Preoperative administration of the 5-HT4 receptor agonist prucalopride reduces intestinal inflammation and shortens postoperative ileus via cholinergic enteric neurons
- PMID: 30472681
- PMCID: PMC6691854
- DOI: 10.1136/gutjnl-2018-317263
Preoperative administration of the 5-HT4 receptor agonist prucalopride reduces intestinal inflammation and shortens postoperative ileus via cholinergic enteric neurons
Abstract
Objectives: Vagus nerve stimulation (VNS), most likely via enteric neurons, prevents postoperative ileus (POI) by reducing activation of alpha7 nicotinic receptor (α7nAChR) positive muscularis macrophages (mMφ) and dampening surgery-induced intestinal inflammation. Here, we evaluated if 5-HT4 receptor (5-HT4R) agonist prucalopride can mimic this effect in mice and human.
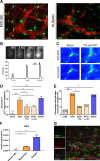
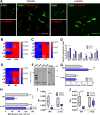
Design: Using Ca2+ imaging, the effect of electrical field stimulation (EFS) and prucalopride was evaluated in situ on mMφ activation evoked by ATP in jejunal muscularis tissue. Next, preoperative and postoperative administration of prucalopride (1-5 mg/kg) was compared with that of preoperative VNS in a model of POI in wild-type and α7nAChR knockout mice. Finally, in a pilot study, patients undergoing a Whipple procedure were preoperatively treated with prucalopride (n=10), abdominal VNS (n=10) or sham/placebo (n=10) to evaluate the effect on intestinal inflammation and clinical recovery of POI.
Results: EFS reduced the ATP-induced Ca2+ response of mMφ, an effect that was dampened by neurotoxins tetrodotoxin and ω-conotoxin and mimicked by prucalopride. In vivo, prucalopride administered before, but not after abdominal surgery reduced intestinal inflammation and prevented POI in wild-type, but not in α7nAChR knockout mice. In humans, preoperative administration of prucalopride, but not of VNS, decreased Il6 and Il8 expression in the muscularis externa and improved clinical recovery.
Conclusion: Enteric neurons dampen mMφ activation, an effect mimicked by prucalopride. Preoperative, but not postoperative treatment with prucalopride prevents intestinal inflammation and shortens POI in both mice and human, indicating that preoperative administration of 5-HT4R agonists should be further evaluated as a treatment of POI.
Trial registration number: NCT02425774.
Keywords: Macrophages; anti-inflammatory; enteric neuron; ileus; prucalopride.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures



Comment in
-
Prucalopride before surgery alleviates postoperative ileus.Nat Rev Gastroenterol Hepatol. 2019 Feb;16(2):76. doi: 10.1038/s41575-019-0106-1. Nat Rev Gastroenterol Hepatol. 2019. PMID: 30643225 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
