Spatial and Temporal Organization of the Individual Human Cerebellum
- PMID: 30473014
- PMCID: PMC6351081
- DOI: 10.1016/j.neuron.2018.10.010
Spatial and Temporal Organization of the Individual Human Cerebellum
Abstract
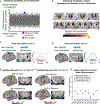
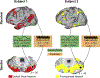
The cerebellum contains the majority of neurons in the human brain and is unique for its uniform cytoarchitecture, absence of aerobic glycolysis, and role in adaptive plasticity. Despite anatomical and physiological differences between the cerebellum and cerebral cortex, group-average functional connectivity studies have identified networks related to specific functions in both structures. Recently, precision functional mapping of individuals revealed that functional networks in the cerebral cortex exhibit measurable individual specificity. Using the highly sampled Midnight Scan Club (MSC) dataset, we found the cerebellum contains reliable, individual-specific network organization that is significantly more variable than the cerebral cortex. The frontoparietal network, thought to support adaptive control, was the only network overrepresented in the cerebellum compared to the cerebral cortex (2.3-fold). Temporally, all cerebellar resting state signals lagged behind the cerebral cortex (125-380 ms), supporting the hypothesis that the cerebellum engages in a domain-general function in the adaptive control of all cortical processes.
Keywords: fMRI; frontoparietal network; functional networks; human cerebellum; individual variability; resting state functional connectivity; temporal lags.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures






Comment in
-
The Big Reveal: Precision Mapping Shines a Gigantic Floodlight on the Cerebellum.Neuron. 2018 Nov 21;100(4):773-776. doi: 10.1016/j.neuron.2018.11.013. Neuron. 2018. PMID: 30465762
References
-
- Andersen BB, Korbo L, and Pakkenberg B (1992). A quantitative study of the human cerebellum with unbiased stereological techniques. J Comp Neurol 326, 549–560. - PubMed
-
- Azizi SA, Burne RA, and Woodward DJ (1985). The auditory corticopontocerebellar projection in the rat: inputs to the paraflocculus and midvermis. An anatomical and physiological study. Exp Brain Res 59, 36–49. - PubMed
-
- Balota DA, Yap MJ, Cortese MJ, Hutchison KA, Kessler B, Loftis B, Neely JH, Nelson DL, Simpson GB, and Treiman R (2007). The English Lexicon Project. Behav Res Methods 39, 445–459. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

