Informing randomized clinical trials of respiratory syncytial virus vaccination during pregnancy to prevent recurrent childhood wheezing: A sample size analysis
- PMID: 30473186
- PMCID: PMC6288067
- DOI: 10.1016/j.vaccine.2018.10.041
Informing randomized clinical trials of respiratory syncytial virus vaccination during pregnancy to prevent recurrent childhood wheezing: A sample size analysis
Abstract
Background: Early RSV illness is associated with wheeze-associated disorders in childhood. Candidate respiratory syncytial virus (RSV) vaccines may prevent acute RSV illness in infants. We investigated the feasibility of maternal RSV vaccine trials to demonstrate reductions in recurrent childhood wheezing in general paediatric populations.
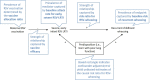
Methods: We calculated vaccine trial effect sizes that depended on vaccine efficacy, allocation ratio, rate of early severe RSV illness, risk of recurrent wheezing at age 3, and increased risk of RSV infection on recurrent wheezing. Model inputs came from systematic reviews and meta-analyses. For each combination of inputs, we estimated the sample size required to detect the effect of vaccination on recurrent wheezing.
Results: There were 81 scenarios with 1:1 allocation ratio. Risk ratios between vaccination and recurrent wheezing ranged from 0.9 to 1.0 for 70% of the scenarios. Among the 57 more plausible scenarios, the lowest sample size required to detect significant reductions in recurrent wheezing was 6196 mother-infant pairs per trial arm; however, 75% and 47% of plausible scenarios required >31,060 and >100,000 mother-infant pairs per trial arm, respectively. Studies with asthma endpoints at age 5 will likely need to be larger.
Discussion: Clinical efficacy trials of candidate maternal RSV vaccines undertaken for licensure are unlikely to demonstrate an effect on recurrent wheezing illness due to the large sample sizes likely needed to demonstrate a significant effect. Further efforts are needed to plan for alternative study designs to estimate the impact of maternal RSV vaccine programs on recurrent childhood wheezing in general populations.
Keywords: Asthma; Global health; Pregnant; Respiratory syncytial virus; Vaccine; Wheeze.
Copyright © 2018 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures



References
-
- World Health Organization. Preferred Product Characteristics for Respiratory Syncytial Virus (RSV) Vaccines. Geneva, Switzerland: World Health Organization; 2017.
-
- Vekemans J., Moorthy V., Giersing B., Friede M., Hombach J., Arora N. Respiratory syncytial virus vaccine research and development: World Health Organization technological roadmap and preferred product characteristics. Vaccine. 2018 - PubMed
-
- Shi T., McAllister D.A., O'Brien K.L., Simoes E.A.F., Madhi S.A., Gessner B.D. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet (London, England) 2017;390:946–958. - PMC - PubMed
-
- Glezen W.P., Taber L.H., Frank A.L., Kasel J.A. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140:543–546. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

