Neuroimaging Applications in Dystonia
- PMID: 30473192
- PMCID: PMC6331056
- DOI: 10.1016/bs.irn.2018.09.007
Neuroimaging Applications in Dystonia
Abstract
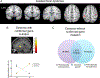
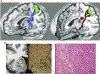
Dystonia is a neurological disorder characterized by involuntary, repetitive movements. Although the precise mechanisms of dystonia development remain unknown, the diversity of its clinical phenotypes is thought to be associated with multifactorial pathophysiology, which is linked not only to alterations of brain organization, but also environmental stressors and gene mutations. This chapter will present an overview of the pathophysiology of isolated dystonia through the lens of applications of major neuroimaging methodologies, with links to genetics and environmental factors that play a prominent role in symptom manifestation.
Keywords: Diffusion imaging; Functional magnetic resonance imaging; Isolated dystonia; Neuroreceptor mapping; Pathophysiology; Positron emission tomography; Voxel-based morphometry.
© 2018 Elsevier Inc. All rights reserved.
Figures
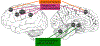


References
-
- Ali SO, Thomassen M, Schulz GM, Hosey LA, Varga M, Ludlow CL, et al. (2006). Alterations in CNS activity induced by botulinum toxin treatment in spasmodic dysphonia: An H215O PET study. Journal of Speech, Language, and Hearing Research: JSLHR, 49(5), 1127–1146. 10.1044/1092-4388(2006/081). - DOI - PubMed
-
- Asanuma K, Ma Y, Okulski J, Dhawan V, Chaly T, Carbon M, et al. (2005). Decreased striatal D2 receptor binding in non-manifesting carriers of the DYT1 dystonia mutation. Neurology, 64(2), 347–349. 10.1212/01.WNL.0000149764.34953.BF. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

