A Cooperative Ternary Catalysis System for Asymmetric Lactonizations of α-Ketoesters
- PMID: 30473657
- PMCID: PMC6251415
- DOI: 10.1002/adsc.201701015
A Cooperative Ternary Catalysis System for Asymmetric Lactonizations of α-Ketoesters
Abstract
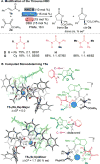
A general and enantioselective N-heterocyclic carbene (NHC)-catalyzed lactonization of simple enals and α-ketoesters has been discovered using a new ternary cooperative catalytic system. The highly selective annulation was achieved by using a combination of a chiral NHC, a hydrogen-bond donor, and a metal salt, facilitating self-assembly of the reactive partners. A proposed model for this new mode of NHC chiral relay catalysis is supported by experimental and computational mechanistic studies.
Keywords: N-heterocyclic carbene; NHC; Umpolung; cooperative catalysis; homoenolate; lactone; ternary catalysis.
Figures


Similar articles
-
Cooperative N-Heterocyclic Carbene/Palladium-Catalyzed Enantioselective Umpolung Annulations.J Am Chem Soc. 2016 Jun 29;138(25):7840-3. doi: 10.1021/jacs.6b04364. Epub 2016 Jun 15. J Am Chem Soc. 2016. PMID: 27266669
-
A cooperative N-heterocyclic carbene/chiral phosphate catalysis system for allenolate annulations.Angew Chem Int Ed Engl. 2014 Jul 14;53(29):7594-8. doi: 10.1002/anie.201403446. Epub 2014 Jun 4. Angew Chem Int Ed Engl. 2014. PMID: 24895280 Free PMC article.
-
Chemoselective Umpolung of Enals for Asymmetric Homoenolate Cross-Annulation of Enals and Aldehydes Catalyzed by N-Heterocyclic Carbene.Org Lett. 2019 Nov 15;21(22):9119-9123. doi: 10.1021/acs.orglett.9b03509. Epub 2019 Nov 5. Org Lett. 2019. PMID: 31686515
-
N-Heterocyclic Carbene (NHC)/Metal Cooperative Catalysis.Top Curr Chem (Cham). 2019 Nov 13;377(6):35. doi: 10.1007/s41061-019-0266-z. Top Curr Chem (Cham). 2019. PMID: 31720874 Review.
-
Chiral N-Heterocyclic Carbene Ligands Enable Asymmetric C-H Bond Functionalization.Angew Chem Int Ed Engl. 2020 Jun 22;59(26):10242-10251. doi: 10.1002/anie.201911898. Epub 2020 Apr 7. Angew Chem Int Ed Engl. 2020. PMID: 31815333 Review.
Cited by
-
A Cooperative Hydrogen Bond Donor-Brønsted Acid System for the Enantioselective Synthesis of Tetrahydropyrans.Angew Chem Int Ed Engl. 2018 Dec 21;57(52):17225-17229. doi: 10.1002/anie.201811383. Epub 2018 Nov 27. Angew Chem Int Ed Engl. 2018. PMID: 30380196 Free PMC article.
-
N-Heterocyclic carbene based catalytic platform for Hauser-Kraus annulations.Chem Sci. 2020 Jun 30;11(27):7239-7243. doi: 10.1039/d0sc03116j. Chem Sci. 2020. PMID: 34123009 Free PMC article.
-
A Sequential Umpolung/Enzymatic Dynamic Kinetic Resolution Strategy for the Synthesis of γ-Lactones.Chemistry. 2020 May 7;26(26):5794-5798. doi: 10.1002/chem.202000747. Epub 2020 Apr 24. Chemistry. 2020. PMID: 32084294 Free PMC article.
References
-
-
For recent NHC reviews, see:
- Nair V, Menon RS, Biju AT, Sinu CR, Paul RR, Jose A, Sreekumar V. Chem Soc Rev. 2011;40:5336–5346. - PubMed
- Bugaut X, Glorius F. Chem Soc Rev. 2012;41:3511–3522. - PubMed
- Hopkinson MN, Richter C, Schedler M, Glorius F. Nature. 2014;510:485–496. - PubMed
- Menon RS, Biju AT, Nair V. Chem Soc Rev. 2015;44:5040–5052. - PubMed
- Flanigan DM, Romanov-Michailidis F, White NA, Rovis T. Chem Rev. 2015;115:9307–9387. - PMC - PubMed
-
-
-
For manipulations of γ-butyrolactones and their appearance in natural or bioactive products, see:
- S’kof M, Svete J, Kmetič M, Golič-Grdadolnik S, Stanovnik B. Eur J Org Chem. 1999:1581–1584.
- Pirc S, Rečnik S, Škof M, Svete J, Golič L, Meden A, Stanovnik B. J Heterocyclic Chem. 2002;39:411–416.
- Miranda PO, Estévez F, Quintana J, García CI, Brouard I, Padrón JI, Pivel JP, Bermejo J. J Med Chem. 2004;47:292–295. - PubMed
- Chimenti F, Cottiglia F, Bonsignore L, Casu L, Casu M, Floris C, Secci D, Bolasco A, Chimenti P, Granese A, Befani O, Turini P, Alcaro S, Ortuso F, Trombetta G, Loizzo A, Guarino I. J Nat Prod. 2006;69:945–949. - PubMed
- Xu HC, Brandt JD, Moeller KD. Tetrahedron Lett. 2008;49:3868–3871.
- Nickerson LA, Huynh V, Balmond EI, Cramer SP, Shaw JT. J Org Chem. 2016;81:11404–11408. - PMC - PubMed
-
-
- Suh H, Wilcox CS. J Am Chem Soc. 1988;110:470–481.
- Reissig HU, Holzinger H, Glomsda G. Tetrahedron. 1989;45:3139–3150.
- Murata Y, Kamino T, Aoki T, Hosokawa S, Kobayashi S. Angew Chem Int Ed. 2004;43:3175–3177. - PubMed
- Zhu Y, Zhai C, Yang L, Hu W. Chem Commun. 2010;46:2865–2867. - PubMed
- Lee A, Scheidt KA. Angew Chem Int Ed. 2014;53:7594–7598. - PMC - PubMed
-
- Dean FM. In: Adv Heterocycl Chem. Katritzky AR, editor. Vol. 30. Academic Press; 1982. pp. 167–238.
-
- Jõgi A, Ilves M, Paju A, Pehk T, Kailas T, Müürisepp A-M, Lopp M. Tetrahedron: Asymmetry. 2008;19:628–634.
- Nakatsuji H, Sawamura Y, Sakakura A, Ishihara K. Angew Chem Int Ed. 2014;53:6974–6977. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
