Quantitative Characterization of the Hemorrhagic, Necrotic, Coagulation-Altering Properties and Edema-Forming Effects of Zebra Snake (Naja nigricincta nigricincta) Venom
- PMID: 30473709
- PMCID: PMC6220379
- DOI: 10.1155/2018/6940798
Quantitative Characterization of the Hemorrhagic, Necrotic, Coagulation-Altering Properties and Edema-Forming Effects of Zebra Snake (Naja nigricincta nigricincta) Venom
Abstract
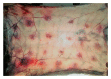
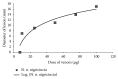
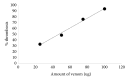
This study was designed to investigate the cytotoxicity and haemotoxicity of the Western barred (zebra) spitting cobra (Naja nigricincta nigricincta) venom to help explain atypical and inconsistent reports on syndromes by Namibian physicians treating victims of human ophidian accidents. Freeze-dried venom milked from adult zebra snakes was dissolved in phosphate buffered saline (PBS) for use in this study. Haemorrhagic and necrotic activity of venom were studied in New Zealand albino rabbits. Oedema-forming activity was investigated in 10-day-old Cobb500 broiler chicks. Procoagulant and thrombolytic activity was investigated in adult Kalahari red goat blood in vitro. The rabbit skin minimum hemorrhagic dose (MHD) for N. n. nigricincta was 9.8μg. The minimum necrotizing dose (MND) for N. n. nigricincta venom was 12.2μg. The N. n. nigricincta venom showed linear dose-dependent procoagulant activity on goat blood (p<0.05). Likewise, N. n. nigricincta venom showed linear dose-dependent thrombolytic activity on goat blood (p<0.05, n = 6). Subplantar injection of N. n. nigricincta venom (25μg, 50μg, 75μg, and 100μg) into chick paw resulted in peak oedema of 35.5%, 38.5%, 42.9%, and 47.5%, respectively, two hours after injection. Paw oedema subsided within five hours to a mean volume ranging from 5% (25μg venom) to 17.6% (100μg venom). In conclusion, though N. n. nigricincta belongs to the genus Elapidae, the current study has shown its venom to possess potent hemorrhagic, necrotic (cytotoxic), and paradoxically, both procoagulant and thrombolytic activity. The authors propose further work to fractionate, isolate, and elucidate the structure of the various N. n. nigricincta venom toxins as a prelude to the development of an antivenom.
Figures




Similar articles
-
Protein Identification of Venoms of the African Spitting Cobras, Naja mossambica and Naja nigricincta nigricincta.Toxins (Basel). 2020 Aug 14;12(8):520. doi: 10.3390/toxins12080520. Toxins (Basel). 2020. PMID: 32823821 Free PMC article.
-
Namibian spitting cobra, Naja nigricincta nigricincta (Zebra snake): Antibiotic profile of bacteria cultured from the oral pharynx, venom and snakebite wounds.S Afr Med J. 2023 Jul 5;113(7):22-28. doi: 10.7196/SAMJ.2023.v113i7.271. S Afr Med J. 2023. PMID: 37882042
-
Prospect of using ethnobotanicals to manage snakebites in a cost-effective manner: validating Senegalia mellifera extract's inhibitory potential on Naja nigricincta nigricincta (zebra cobra) venom.Trans R Soc Trop Med Hyg. 2025 Feb 4;119(2):166-174. doi: 10.1093/trstmh/trae055. Trans R Soc Trop Med Hyg. 2025. PMID: 39749495
-
Naja nigricincta nigricincta venom, a murine model. Evaluation of skeletal and cardio-myonecrosis, kidney injury and inflammatory response along with neutralisation efficacy by the SAIMR/SAVP - And EchiTAb-Plus-ICP polyvalent antivenoms.Toxicon. 2024 May 28;243:107719. doi: 10.1016/j.toxicon.2024.107719. Epub 2024 Apr 15. Toxicon. 2024. PMID: 38631492
-
Protective activity of medicinal plants and their isolated compounds against the toxic effects from the venom of Naja (cobra) species.J Ethnopharmacol. 2014 Nov 18;157:222-7. doi: 10.1016/j.jep.2014.09.039. Epub 2014 Oct 5. J Ethnopharmacol. 2014. PMID: 25291011 Review.
Cited by
-
Coagulotoxic Cobras: Clinical Implications of Strong Anticoagulant Actions of African Spitting Naja Venoms That Are Not Neutralised by Antivenom but Are by LY315920 (Varespladib).Toxins (Basel). 2018 Dec 4;10(12):516. doi: 10.3390/toxins10120516. Toxins (Basel). 2018. PMID: 30518149 Free PMC article.
-
The Relative Efficacy of Chemically Diverse Small-Molecule Enzyme-Inhibitors Against Anticoagulant Activities of African Spitting Cobra (Naja Species) Venoms.Front Immunol. 2021 Oct 7;12:752442. doi: 10.3389/fimmu.2021.752442. eCollection 2021. Front Immunol. 2021. PMID: 34691069 Free PMC article.
-
Evaluation of lethality and cytotoxic effects induced by Naja ashei (large brown spitting cobra) venom and the envenomation-neutralizing efficacy of selected commercial antivenoms in Kenya.Toxicon X. 2022 May 4;14:100125. doi: 10.1016/j.toxcx.2022.100125. eCollection 2022 Jun. Toxicon X. 2022. PMID: 35574180 Free PMC article.
-
Dermonecrosis caused by a spitting cobra snakebite results from toxin potentiation and is prevented by the repurposed drug varespladib.Proc Natl Acad Sci U S A. 2024 May 7;121(19):e2315597121. doi: 10.1073/pnas.2315597121. Epub 2024 Apr 30. Proc Natl Acad Sci U S A. 2024. PMID: 38687786 Free PMC article.
-
Protein Identification of Venoms of the African Spitting Cobras, Naja mossambica and Naja nigricincta nigricincta.Toxins (Basel). 2020 Aug 14;12(8):520. doi: 10.3390/toxins12080520. Toxins (Basel). 2020. PMID: 32823821 Free PMC article.
References
-
- Hirebail S. K., Nagabushan H., Prakash G. M. A prospective study of efficacy and safety of olopatadine versus azelastine in allergic conjunctivitis at a tertiary care hospital. International Journal of Basic & Clinical Pharmacology. 2017;6(12):2836–2842. doi: 10.18203/2319-2003.ijbcp20173276. - DOI
-
- WHO. WHO guidelines for the production, control and regulation of snake antivenom immunoglobulins. Geneva, vol. 134, 2010. - PubMed
-
- Wood D., Webb C., Demeyer J. Severe snakebites in northern KwaZulu-Natal: Treatment modalities and outcomes. South African Medical Journal. 2009;99(11):814–818. - PubMed
LinkOut - more resources
Full Text Sources

