The role of neoantigen in immune checkpoint blockade therapy
- PMID: 30473928
- PMCID: PMC6240277
- DOI: 10.1186/s40164-018-0120-y
The role of neoantigen in immune checkpoint blockade therapy
Abstract
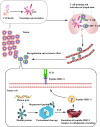
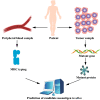
Immune checkpoint inhibitor induces tumor rejection by activated host immune system. The anti-tumor immune response consists of capture, presentation, recognition of neoantigen, as well as subsequent killing of tumor cell. Due to the interdependence among this series of stepwise events, neoantigen profoundly influences the efficacy of anti-immune checkpoint therapy. Moreover, the neoantigen-specific T cell reactivity is the cornerstone of multiple immunotherapies. In fact, several strategies targeting neoantigen have been attempted for synergetic effect with immune checkpoint inhibitor. Increasing neoantigen presentation to immune system by oncolytic virus, radiotherapy, or cancer vaccine is feasible to enhance neoantigen-specific T cell reactivity in theory. However, some obstacles have not been overcome in practice such as dynamic variation of neoantigen landscape, identification of potential neoantigen, maintenance of high T cell titer post vaccination. In addition, adoptive T cell transfer is another approach to enhance neoantigen-specific T cell reactivity, especially for patients with severe immunosuppression. In this review, we highlighted the advancements of neoantigen and innovative explorations of utilization of neoantigen repertoire in immune checkpoint blockade therapy.
Keywords: Adoptive T cell transfer; CTLA-4; Cancer vaccine; Immunotherapy; Neoantigen; PD-1/PD-L1.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

