Isotretinoin exposure in pregnant women in Korea
- PMID: 30474010
- PMCID: PMC6236094
- DOI: 10.5468/ogs.2018.61.6.649
Isotretinoin exposure in pregnant women in Korea
Abstract
Objective: Isotretinoin is a notorious teratogen otherwise used for the treatment of acne vulgaris. Some countries, including those in North America and the European Union, implemented the pregnancy prevention program (PPP); however, no PPP has yet been established in South Korea. So the aim of this study was to evaluate the rate of pregnant women exposed to isotretinoin among the callers of the Korean Mother Safe Counseling Center.
Methods: This is a prospective cohort study. We evaluated the demographic characteristics, obstetric history, and isotretinoin exposure of pregnant women based on the mother safe registry from April 2010 to July 2016.
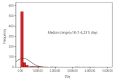
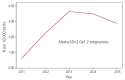
Results: Among 22,374 callers, 650 (2.9%) pregnant women were exposed to isotretinoin. The mean age was 29.0±4.4 years in the isotretinoin-exposed group and 32.0±4.2 years in the unexposed group (P<0.001). Moreover, the incidence of pregnancies within 30 days after isotretinoin discontinuation or during isotretinoin intake was 78.9% (513/650). The median duration of isotretinoin exposure was 18 (1-4,231) days. Furthermore, from 2011 to 2015, the incidence of isotretinoin exposure was 2.9±1.2 pregnancies per 10,000 births in South Korea.
Conclusion: Approximately 80% of pregnant women are exposed to isotretinoin within the recommended 30 days of contraception or during pregnancy. Therefore, the PPP has to be established in South Korea.
Keywords: Isotretinoin; Pregnancy; Teratogen.
Conflict of interest statement
Conflict of interest: No potential conflict of interest relevant to this article was reported.
Figures
References
-
- Korea Pharmaceutical Information Center. Isotretinoin information [Internet] Seoul: Korea Pharmaceutical Information Center; c2018. [cited 2018 Feb 19]. Available from: http://www.health.kr/searchDrug/result_drug.asp?drug_cd=A11AHHHHH0304.
-
- Azoulay L, Oraichi D, Bérard A. Patterns and utilization of isotretinoin for acne from 1984 to 2003: is there need for concern? Eur J Clin Pharmacol. 2006;62:667–674. - PubMed
-
- Honein MA, Paulozzi LJ, Erickson JD. Continued occurrence of Accutane-exposed pregnancies. Teratology. 2001;64:142–147. - PubMed
-
- Lammer EJ, Chen DT, Hoar RM, Agnish ND, Benke PJ, Braun JT, et al. Retinoic acid embryopathy. N Engl J Med. 1985;313:837–841. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous