The Inhibition Effect of Tert-Butyl Alcohol on the TiO2 Nano Assays Photoelectrocatalytic Degradation of Different Organics and Its Mechanism
- PMID: 30474033
- PMCID: PMC6225928
- DOI: 10.1007/s40820-015-0080-2
The Inhibition Effect of Tert-Butyl Alcohol on the TiO2 Nano Assays Photoelectrocatalytic Degradation of Different Organics and Its Mechanism
Abstract
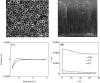
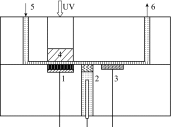
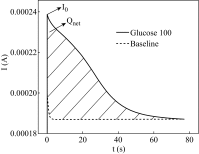
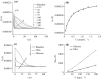
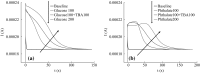
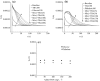
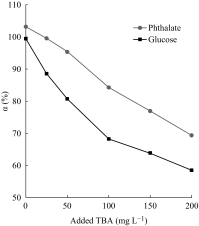
The inhibition effect of tert-butyl alcohol (TBA), identified as the •OH radical inhibitor, on the TiO2 nano assays (TNA) photoelectrocatalytic oxidation of different organics such as glucose and phthalate was reported. The adsorption performance of these organics on the TNA photoelectrode was investigated by using the instantaneous photocurrent value, and the degradation property was examined by using the exhausted reaction. The results showed that glucose exhibited the poor adsorption and easy degradation performance, phthalate showed the strong adsorption and hard-degradation, but TBA showed the weak adsorption and was the most difficult to be degraded. The degradation of both glucose and phthalate could be inhibited evidently by TBA. But the effect on glucose was more obvious. The different inhibition effects of TBA on different organics could be attributed to the differences in the adsorption and the degradation property. For instance, phthalate of the strong adsorption property could avoid from the capture of •OH radicals by TBA in TNA photoelectrocatalytic process.
Keywords: Hydroxyl radical inhibitor; Inhibition effect; Photoelectrocatalysis; Tert-butyl alcohol; TiO2 nano assays.
Figures


References
-
- Zhang XW, Wang DK, Diniz Da Costa JC. Recent progresses on fabrication of photocatalytic membranes for water treatment. Catal. Today. 2014;230:47–54. doi: 10.1016/j.cattod.2013.11.019. - DOI
-
- Teh CM, Mohamed AR. Roles of titanium dioxide and ion-doped titanium dioxide on photocatalytic degradation of organic pollutants (phenolic compounds and dyes) in aqueous solutions: a review. J. Alloy. Compd. 2011;509(5):1648–1660. doi: 10.1016/j.jallcom.2010.10.181. - DOI
-
- Wei H, Wang L, Li Zh, Ni S, Zhao Q. Synthesis and photocatalytic activity of one-dimensional CdS@TiO2core-shell heterostructures. Nano-Micro Lett. 2011;3(1):6–11. doi: 10.1007/BF03353645. - DOI
-
- Nakata K, Fujishima A. TiO2photocatalysis: design and applications. J. Photochem. Photobiol. C. 2012;13(3):169–189. doi: 10.1016/j.jphotochemrev.2012.06.001. - DOI
LinkOut - more resources
Full Text Sources
