Microbiological testing of adults hospitalised with community-acquired pneumonia: an international study
- PMID: 30474036
- PMCID: PMC6174282
- DOI: 10.1183/23120541.00096-2018
Microbiological testing of adults hospitalised with community-acquired pneumonia: an international study
Abstract
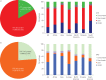
This study aimed to describe real-life microbiological testing of adults hospitalised with community-acquired pneumonia (CAP) and to assess concordance with the 2007 Infectious Diseases Society of America (IDSA)/American Thoracic Society (ATS) and 2011 European Respiratory Society (ERS) CAP guidelines. This was a cohort study based on the Global Initiative for Methicillin-resistant Staphylococcus aureus Pneumonia (GLIMP) database, which contains point-prevalence data on adults hospitalised with CAP across 54 countries during 2015. In total, 3702 patients were included. Testing was performed in 3217 patients, and included blood culture (71.1%), sputum culture (61.8%), Legionella urinary antigen test (30.1%), pneumococcal urinary antigen test (30.0%), viral testing (14.9%), acute-phase serology (8.8%), bronchoalveolar lavage culture (8.4%) and pleural fluid culture (3.2%). A pathogen was detected in 1173 (36.5%) patients. Testing attitudes varied significantly according to geography and disease severity. Testing was concordant with IDSA/ATS and ERS guidelines in 16.7% and 23.9% of patients, respectively. IDSA/ATS concordance was higher in Europe than in North America (21.5% versus 9.8%; p<0.01), while ERS concordance was higher in North America than in Europe (33.5% versus 19.5%; p<0.01). Testing practices of adults hospitalised with CAP varied significantly by geography and disease severity. There was a wide discordance between real-life testing practices and IDSA/ATS/ERS guideline recommendations.
Conflict of interest statement
Conflict of interest: M. Carugati has nothing to disclose. Conflict of interest: S. Aliberti reports grants and personal fees from Bayer Healthcare, Aradigm Corporation, Grifols, Chiesi and Insmed, and personal fees from AstraZeneca, Basilea, Zambon, Novartis, Raptor, Actavis UK Ltd and Horizon, all outside the submitted work. Conflict of interest: L.F. Reyes has nothing to disclose. Conflict of interest: R. Franco Sadud has nothing to disclose. Conflict of interest: M. Irfan has nothing to disclose. Conflict of interest: C. Prat has nothing to disclose. Conflict of interest: N.J. Soni has nothing to disclose. Conflict of interest: P. Faverio has nothing to disclose. Conflict of interest: A. Gori reports receipt of grants/research support from Abbvie, Astellas, BMS, Boehringer, Gilead, Janssen, MSD, Novartis, Pfizer, Roche, ViiV and ANLAIDS Sezione Lombarda, receipt of honoraria or consultation fees from BMS, Gilead, Janssen, MSD, Novartis and ViiV, participation in a company sponsored speakers’ bureau from Gilead, and travel grants/support from BMS, Gilead, Janssen and ViiV. Conflict of interest: F. Blasi reports personal fees from AstraZeneca, Guidotti Malesci, GSK and Novartis, and grants and personal fees from Bayer and Chiesi, Grifols, Insmed, Menarini, Pfizer, Teva and Zambon, all outside the submitted work. Conflict of interest: M.I. Restrepo has nothing to disclose.
Figures
References
-
- Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med 1997; 336: 243–250. - PubMed
-
- Johnstone J, Eurich DT, Majumdar SR, et al. Long-term morbidity and mortality after hospitalization with community-acquired pneumonia: a population-based cohort study. Medicine 2008; 87: 329–334. - PubMed
-
- Metersky ML, Waterer G, Nsa W, et al. Predictors of in-hospital vs postdischarge mortality in pneumonia. Chest 2012; 142: 476–481. - PubMed
