A central control circuit for encoding perceived food value
- PMID: 30474061
- PMCID: PMC6248929
- DOI: 10.1126/sciadv.aau9180
A central control circuit for encoding perceived food value
Abstract
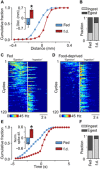
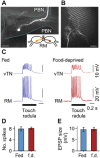
Hunger state can substantially alter the perceived value of a stimulus, even to the extent that the same sensory cue can trigger antagonistic behaviors. How the nervous system uses these graded perceptual shifts to select between opposed motor patterns remains enigmatic. Here, we challenged food-deprived and satiated Lymnaea to choose between two mutually exclusive behaviors, ingestion or egestion, produced by the same feeding central pattern generator. Decoding the underlying neural circuit reveals that the activity of central dopaminergic interneurons defines hunger state and drives network reconfiguration, biasing satiated animals toward the rejection of stimuli deemed palatable by food-deprived ones. By blocking the action of these neurons, satiated animals can be reconfigured to exhibit a hungry animal phenotype. This centralized mechanism occurs in the complete absence of sensory retuning and generalizes across different sensory modalities, allowing food-deprived animals to increase their perception of food value in a stimulus-independent manner to maximize potential calorific intake.
Figures




References
-
- Gaudry Q., Kristan W. B. Jr., Behavioral choice by presynaptic inhibition of tactile sensory terminals. Nat. Neurosci. 12, 1450–1457 (2009). - PubMed
-
- Filosa A., Barker A. J., Dal Maschio M., Baier H., Feeding state modulates behavioral choice and processing of prey stimuli in the zebrafish tectum. Neuron 90, 596–608 (2016). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

