Accrual to Clinical Trials (ACT): A Clinical and Translational Science Award Consortium Network
- PMID: 30474072
- PMCID: PMC6241502
- DOI: 10.1093/jamiaopen/ooy033
Accrual to Clinical Trials (ACT): A Clinical and Translational Science Award Consortium Network
Abstract
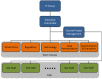
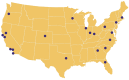
The Accrual to Clinical Trials (ACT) network is a federated network of sites from the National Clinical and Translational Science Award (CTSA) Consortium that has been created to significantly increase participant accrual to multi-site clinical trials. The ACT network represents an unprecedented collaboration among diverse CTSA sites. The network has created governance and regulatory frameworks and a common data model to harmonize electronic health record (EHR) data, and deployed a set of Informatics for Integrating Biology and the Bedside (i2b2) data repositories that are linked by the Shared Health Research Information Network (SHRINE) platform. It provides investigators the ability to query the network in real time and to obtain aggregate counts of patients who meet clinical trial inclusion and exclusion criteria from sites across the United States. The ACT network infrastructure provides a basis for cohort discovery and for developing new informatics tools to identify and recruit participants for multi-site clinical trials.
Keywords: accrual; clinical data research network; clinical trials; cohort discovery; electronic health records.
Figures

References
-
- Irving SY, Curley MA.. Challenges to conducting multicenter clinical research: ten points to consider. AACN Adv Crit Care 2008; 192: 164–9. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
