A Curated Cancer Clinical Outcomes Database (C3OD) for accelerating patient recruitment in cancer clinical trials
- PMID: 30474074
- PMCID: PMC6241508
- DOI: 10.1093/jamiaopen/ooy023
A Curated Cancer Clinical Outcomes Database (C3OD) for accelerating patient recruitment in cancer clinical trials
Abstract
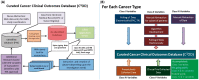
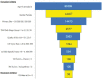
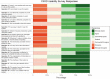
Data used to determine patient eligibility for cancer clinical trials often come from disparate sources that are typically maintained by different groups within an institution, use differing technologies, and are stored in different formats. Collecting data and resolving inconsistencies across sources increase the time it takes to screen eligible patients, potentially delaying study completion. To address these challenges, the Biostatistics and Informatics Shared Resource at The University of Kansas Cancer Center developed the Curated Cancer Clinical Outcomes Database (C3OD). C3OD merges data from the electronic medical record, tumor registry, bio-specimen and data registry, and allows querying through a single unified platform. By centralizing access and maintaining appropriate controls, C3OD allows researchers to more rapidly obtain detailed information about each patient in order to accelerate eligibility screening. This case report describes the design of this informatics platform as well as initial assessments of its reliability and usability.
Keywords: automated eligibility screening; clinical research informatics; patient recruitment.
Figures
References
-
- Ferland D, Fortin PR.. Recruitment strategies in superiority trials in SLE: lessons from the study of methotrexate in lupus erythematosus (SMILE). Lupus 1999; 88: 606–11. - PubMed
-
- Lovato LC, Hill K, Hertert S, et al. Recruitment for controlled clinical trials: literature summary and annotated bibliography. Control Clin Trials 1997; 184: 328–52. - PubMed
-
- Collins JF, Williford WO, Weiss DG, et al. Planning patient recruitment: fantasy and reality. Stat Med 1984; 3: 435–43. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
