Breast cancer-released exosomes trigger cancer-associated cachexia to promote tumor progression
- PMID: 30474469
- PMCID: PMC6768245
- DOI: 10.1080/21623945.2018.1551688
Breast cancer-released exosomes trigger cancer-associated cachexia to promote tumor progression
Retraction in
-
Retraction.Adipocyte. 2023 Dec;12(1):2187568. doi: 10.1080/21623945.2023.2187568. Adipocyte. 2023. PMID: 36961293 Free PMC article. No abstract available.
Abstract
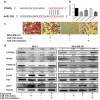
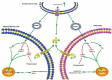
Cancer-secreted exosomes are emerging mediators of cancer-associated cachexia. Here, we show that miR-155 secreted by breast cancer cells is a potent role on the catabolism of adipocytes and muscle cells through targeting the PPARγ. After cocultivated with mature adipocytes or C2C12, tumour cells exhibit an aggressive phenotype via inducing epithelial-mesenchymal transition while breast cancer-derived exosomes increased catabolism and release the metabolites in adipocytes and muscle cells. In adipocytes, cancer cell-secreted miR-155 promotes beige/brown differentiation and remodel metabolism in resident adipocytes by downregulating the PPARγ expression, but does not significantly affect biological conversion in C2C12. Likewise, propranolol ameliorates tumour exosomes-associated cachectic wasting through upregulating the PPARγ expression. In summary, we have demonstrated that the transfer of miR-155 from exosomes acts as an oncogenic signal reprograming systemic energy metabolism and leading to cancer-associated cachexia in breast cancer.
Keywords: Breast cancer; cachexia; exosomes; tumour progression.
Figures





References
-
- Fearon KC, Glass DJ, Guttridge DC.. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 2012. August 8;16(2):153–166. PMID: 22795476. - PubMed
-
- Martinez-Outschoorn U, Sotgia F, Lisanti MP. Tumor microenvironment and metabolic synergy in breast cancers: critical importance of mitochondrial fuels and function. Semin Oncol. 2014. April;41(2):195–216. PMID: 24787293. - PubMed
-
- Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer. 2002. November;2(11):862–871. PMID: 12415256. - PubMed
-
- Bruggeman AR, Kamal AH, LeBlanc TW, Ma JD, Baracos VE, Roeland EJ. Cancer cachexia: beyond weight Loss. J Oncol Pract. 2016. November;12(11):1163–1171. PMID: 27858548. - PubMed
-
- Vigano AAL, Morais JA, Ciutto L, Rosenthall L, Di Tomasso J, Khan S, Olders H, Borod M, Kilgour RD. Use of routinely available clinical, nutritional, and functional criteria to classify cachexia in advanced cancer patients. Clin Nutr. 2017. October;36(5):1378–1390. PMID: 27793524. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
