CITRIC: cold-inducible translational readthrough in the chloroplast of Chlamydomonas reinhardtii using a novel temperature-sensitive transfer RNA
- PMID: 30474564
- PMCID: PMC6260665
- DOI: 10.1186/s12934-018-1033-5
CITRIC: cold-inducible translational readthrough in the chloroplast of Chlamydomonas reinhardtii using a novel temperature-sensitive transfer RNA
Abstract
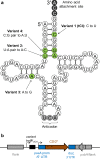
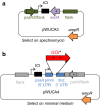
Background: The chloroplast of eukaryotic microalgae such as Chlamydomonas reinhardtii is a potential platform for metabolic engineering and the production of recombinant proteins. In industrial biotechnology, inducible expression is often used so that the translation or function of the heterologous protein does not interfere with biomass accumulation during the growth stage. However, the existing systems used in bacterial or fungal platforms do not transfer well to the microalgal chloroplast. We sought to develop a simple inducible expression system for the microalgal chloroplast, exploiting an unused stop codon (TGA) in the plastid genome. We have previously shown that this codon can be translated as tryptophan when we introduce into the chloroplast genome a trnWUCA gene encoding a plastidial transfer RNA with a modified anticodon sequence, UCA.
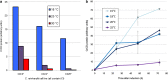
Results: A mutated version of our trnWUCA gene was developed that encodes a temperature-sensitive variant of the tRNA. This allows transgenes that have been modified to contain one or more internal TGA codons to be translated differentially according to the culture temperature, with a gradient of recombinant protein accumulation from 35 °C (low/off) to 15 °C (high). We have named this the CITRIC system, an acronym for cold-inducible translational readthrough in chloroplasts. The exact induction behaviour can be tailored by altering the number of TGA codons within the transgene.
Conclusions: CITRIC adds to the suite of genetic engineering tools available for the microalgal chloroplast, allowing a greater degree of control over the timing of heterologous protein expression. It could also be used as a heat-repressible system for studying the function of essential native genes in the chloroplast. The genetic components of CITRIC are entirely plastid-based, so no engineering of the nuclear genome is required.
Keywords: Chloroplast; Inducible expression; Industrial biotechnology; Microalgae; Opal suppression; Premature termination codon; tRNA.
Figures



References
-
- Gallaher SD, Fitz-Gibbon ST, Strenkert D, Purvine SO, Pellegrini M, Merchant SS. High-throughput sequencing of the chloroplast and mitochondrion of Chlamydomonas reinhardtii to generate improved de novo assemblies, analyze expression patterns and transcript speciation, and evaluate diversity among laboratory strains and wild isolates. Plant J. 2018;93:545–565. doi: 10.1111/tpj.13788. - DOI - PMC - PubMed

