Synthesis, crystal structure, DFT studies and biological activity of (Z)-3-(3-bromophenyl)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxyprop-2-en-1-one
- PMID: 30474759
- PMCID: PMC6768133
- DOI: 10.1186/s13065-018-0492-4
Synthesis, crystal structure, DFT studies and biological activity of (Z)-3-(3-bromophenyl)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxyprop-2-en-1-one
Abstract
Background: Nowadays, is emerging a new generation of highly promising inhibitors bearing the β-ketoenol functionality. The present work relates to the first synthesis, the structure determination, the DFT studies and the use of a new biomolecule designed with a β-ketoenol group bounded to a pyrazolic moiety.
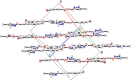
Result: A novel β-ketoenol-pyrazole has been synthesized, well characterized and its structure was confirmed by single crystal X-ray diffraction. The electron densities and the HOMO-LUMO gap have been calculated using the DFT method with BLYP, PW91, PWC functionals and 6-31G* basis set. An evaluation of the molecule stability is provided by a NBO analysis and the calculated Fukui and Parr functions have been used to locate the reactive electrophile and nucleophile centers in the molecule. The synthesized compound, screened for its in vitro antifungal behavior against the Fusarium oxysporum f.sp. albedinis FAO fungal strains, shows a moderate activity with an inhibition percentage of 46%. The product was also tested against three bacterial strains (Escherichia coli, Bacillus subtilis and Micrococcus luteus), but no significant effect was observed against these organisms.
Conclusions: Density functional calculations are used to evaluate the HOMO-LUMO energy gap, the molecular electrostatic potential and to provide a natural bond orbital analysis. The measured antimicrobial activities encourage us to continue searching for other structures, likely to be good antifungal candidates.
Keywords: Biological activity; Fukui and Parr functions; NBO analysis; Reactivity indices; Single-crystal structure; β-Keto-enol-pyrazole.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




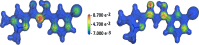

Similar articles
-
An Insight into All Tested Small Molecules against Fusarium oxysporum f. sp. Albedinis: A Comparative Review.Molecules. 2022 Apr 22;27(9):2698. doi: 10.3390/molecules27092698. Molecules. 2022. PMID: 35566050 Free PMC article. Review.
-
X-ray Single Crystal Structure, DFT Calculations and Biological Activity of 2-(3-Methyl-5-(pyridin-2'-yl)-1H-pyrazol-1-yl) Ethanol.Molecules. 2016 Aug 5;21(8):1020. doi: 10.3390/molecules21081020. Molecules. 2016. PMID: 27527141 Free PMC article.
-
Novel β-keto-enol Pyrazolic Compounds as Potent Antifungal Agents. Design, Synthesis, Crystal Structure, DFT, Homology Modeling, and Docking Studies.J Chem Inf Model. 2019 Apr 22;59(4):1398-1409. doi: 10.1021/acs.jcim.8b00828. Epub 2019 Apr 10. J Chem Inf Model. 2019. PMID: 30935197
-
Synthesis, Biochemical Characterization, and Theoretical Studies of Novel β-Keto-enol Pyridine and Furan Derivatives as Potent Antifungal Agents.ACS Omega. 2020 Jul 9;5(28):17743-17752. doi: 10.1021/acsomega.0c02365. eCollection 2020 Jul 21. ACS Omega. 2020. PMID: 32715261 Free PMC article.
-
Synthesis, crystal structure, biological activity and theoretical calculations of novel isoxazole derivatives.Spectrochim Acta A Mol Biomol Spectrosc. 2016 Jan 5;152:226-32. doi: 10.1016/j.saa.2015.07.057. Epub 2015 Jul 10. Spectrochim Acta A Mol Biomol Spectrosc. 2016. PMID: 26218917
Cited by
-
Synthesis, spectroscopy, crystal structure, TGA/DTA study, DFT and molecular docking investigations of (E)-4-(4-methylbenzyl)-6-styrylpyridazin-3(2H)-one.J Mol Struct. 2021 Mar 15;1228:129435. doi: 10.1016/j.molstruc.2020.129435. Epub 2020 Oct 10. J Mol Struct. 2021. PMID: 33071353 Free PMC article.
-
New N-Alkylated Heterocyclic Compounds as Prospective NDM1 Inhibitors: Investigation of In Vitro and In Silico Properties.Pharmaceuticals (Basel). 2022 Jun 28;15(7):803. doi: 10.3390/ph15070803. Pharmaceuticals (Basel). 2022. PMID: 35890102 Free PMC article.
-
An Insight into All Tested Small Molecules against Fusarium oxysporum f. sp. Albedinis: A Comparative Review.Molecules. 2022 Apr 22;27(9):2698. doi: 10.3390/molecules27092698. Molecules. 2022. PMID: 35566050 Free PMC article. Review.
-
Mono-Alkylated Ligands Based on Pyrazole and Triazole Derivatives Tested Against Fusarium oxysporum f. sp. albedinis: Synthesis, Characterization, DFT, and Phytase Binding Site Identification Using Blind Docking/Virtual Screening for Potent Fophy Inhibitors.Front Chem. 2020 Dec 11;8:559262. doi: 10.3389/fchem.2020.559262. eCollection 2020. Front Chem. 2020. PMID: 33363103 Free PMC article.
-
Coordination complexes constructed from pyrazole-acetamide and pyrazole-quinoxaline: effect of hydrogen bonding on the self-assembly process and antibacterial activity.RSC Adv. 2022 Feb 14;12(9):5324-5339. doi: 10.1039/d1ra09027e. eCollection 2022 Feb 10. RSC Adv. 2022. PMID: 35425576 Free PMC article.
References
-
- Dowling C, Murphy VJ, Parkin G. Bis(pyrazolylethyl) ether ligation to zinc and cobalt: meridional vs facial coordination and the suitability of such ligands in providing a NNO donor set for modeling bioinorganic aspects of zinc chemistry. Inorg Chem. 1996;35:2415–2420. doi: 10.1021/ic9512744. - DOI - PubMed
-
- Haanstra WJ, Driessen WL, Van Roon M, Stoffels ALE, Reedijk J. Co-ordination compounds with the N 2 S-donor ligand 1, 5-bis (3, 5-dimethylpyrazol-1-yl)-3-thiapentane. J Chem Soc Dalton Trans. 1992;3:481–486. doi: 10.1039/dt9920000481. - DOI
-
- Driessen WL, Wiesmeijer WGR, Schipper-Zablotskaja M, De Graaff RAG, Reedijk J. Transition metal coordination compounds of two pyrazole-substituted ammonia ligands. X-ray structure of [Biss(1-pyrazolylmethyl)aminecobalt(II)] bis(nitrate) Inorg Chim Acta. 1989;162:233–238. doi: 10.1016/S0020-1693(00)83153-9. - DOI
-
- Pennings YCM, Driessen WL, Reedijk J. Copper(I) coordination compounds with two bidentate chelating pyrazole ligands. X-ray crystal structures of DI-μ-chloro-bis[[N, N-bis(1- pyrazolylmethyl)aminoethane]copper(I)] and [bis(N, N-bis(1-pyrazolylmethyl)aminoethane]copper(I) triflate. Polyhedron. 1988;7:2583–2589. doi: 10.1016/S0277-5387(00)83877-2. - DOI
-
- Veldhuis JB, Driessen WL, Reedijk JA. A pyrazole derivative of aminoethane as a tridentate chelating ligand towards transition metals. The X-ray structure of [N, N-bis(pyrazol-1-ylmethyl)aminoethane]dibromocopper(II. Chem Soc Dalton Trans. 1986;3:537–541. doi: 10.1039/dt9860000537. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

