Wound Healing: A Cellular Perspective
- PMID: 30475656
- PMCID: PMC6442927
- DOI: 10.1152/physrev.00067.2017
Wound Healing: A Cellular Perspective
Abstract
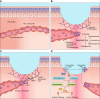
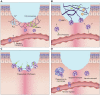
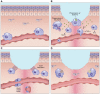
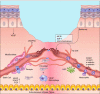
Wound healing is one of the most complex processes in the human body. It involves the spatial and temporal synchronization of a variety of cell types with distinct roles in the phases of hemostasis, inflammation, growth, re-epithelialization, and remodeling. With the evolution of single cell technologies, it has been possible to uncover phenotypic and functional heterogeneity within several of these cell types. There have also been discoveries of rare, stem cell subsets within the skin, which are unipotent in the uninjured state, but become multipotent following skin injury. Unraveling the roles of each of these cell types and their interactions with each other is important in understanding the mechanisms of normal wound closure. Changes in the microenvironment including alterations in mechanical forces, oxygen levels, chemokines, extracellular matrix and growth factor synthesis directly impact cellular recruitment and activation, leading to impaired states of wound healing. Single cell technologies can be used to decipher these cellular alterations in diseased states such as in chronic wounds and hypertrophic scarring so that effective therapeutic solutions for healing wounds can be developed.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

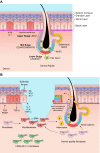
References
-
- Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation 110: 3300–3305, 2004. doi: 10.1161/01.CIR.0000147780.30124.CF. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

