Central adjudication of serious adverse events did not affect trial's safety results: Data from the Efficacy of Nitric Oxide in Stroke (ENOS) trial
- PMID: 30475912
- PMCID: PMC6258247
- DOI: 10.1371/journal.pone.0208142
Central adjudication of serious adverse events did not affect trial's safety results: Data from the Efficacy of Nitric Oxide in Stroke (ENOS) trial
Abstract
Background and purpose: Central adjudication of serious adverse events (SAEs) can be undertaken in clinical trials, especially for open-label studies where outcome assessment may be at risk of bias. This study explored the effect of central adjudication of SAEs on the safety results of the Efficacy of Nitric Oxide in Stroke (ENOS) Trial.
Methods: ENOS assigned patients with acute stroke at random to receive either transdermal glyceryl trinitrate (GTN) or no GTN and to Stop or Continue previous antihypertensive treatment. SAEs were reported by local investigators who were not blinded to treatment allocation. Central adjudicators, blinded to treatment allocation, reviewed the investigators reports and used evidence available to confirm or re-categorise the classification of event, likely causality, diagnosis and expectedness of event.
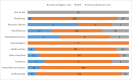
Results: Of 4011 patients enrolled in ENOS, 1473 SAEs were reported by local investigators; this was reduced to 1444 after the review by adjudicators, with 29 re-classified as not an SAE. There was fair agreement between investigators and adjudicators regarding likely causality, with 808 agreements and 644 disagreements (56% crude agreement, weighted kappa, κ = 0.31). Agreement increased upon dichotomisation of the causality categories, with 1432 agreements and 20 disagreements (99% crude agreement, kappa = 0.54). Repeating the main trial safety analysis with investigator reported events showed that adjudication had no effect on the main trial safety conclusions.
Conclusions: In a large trial, with many SAEs reported, central adjudication of these events did not affect trial conclusions. This suggests that adjudication of SAEs in a clinical trial where the intervention already has a well-established safety profile may not be necessary. Potential efficiency savings (financial, logistical) can be made through not adjudicating SAEs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Granger CB, Vogel V, Cummings SR, Held P, Fiedorek F, Lawrence M, et al. Do we need to adjudicate major clinical events? Clinical trials (London, England). 2008;5(1):56–60. Epub 2008/02/20. 10.1177/1740774507087972 . - DOI - PubMed
-
- Pogue J, Walter SD, Yusuf S. Evaluating the benefit of event adjudication of cardiovascular outcomes in large simple RCTs. Clinical trials (London, England). 2009;6(3):239–51. Epub 2009/06/17. 10.1177/1740774509105223 . - DOI - PubMed
-
- Walter SD, Cook DJ, Guyatt GH, King D, Troyan S. Outcome assessment for clinical trials: how many adjudicators do we need? Canadian Lung Oncology Group. Controlled clinical trials. 1997;18(1):27–42. Epub 1997/02/01. . - PubMed
-
- Hrobjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomised clinical trials with binary outcomes: systematic review of trials with both blinded and non-blinded outcome assessors. BMJ (Clinical research ed). 2012;344:e1119 Epub 2012/03/01. 10.1136/bmj.e1119 . - DOI - PubMed
-
- Hrobjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors. CMAJ: Canadian Medical Association journal = journal de l'Association medicale canadienne. 2013;185(4):E201–11. Epub 2013/01/30. 10.1503/cmaj.120744 ; PubMed Central PMCID: PMCPMC3589328. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical