Determinants of new wavefront locations in cholinergic atrial fibrillation
- PMID: 30476057
- PMCID: PMC6251188
- DOI: 10.1093/europace/euy235
Determinants of new wavefront locations in cholinergic atrial fibrillation
Abstract
Aims: Atrial fibrillation (AF) wavefront dynamics are complex and difficult to interpret, contributing to uncertainty about the mechanisms that maintain AF. We aimed to investigate the interplay between rotors, wavelets, and focal sources during fibrillation.
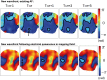
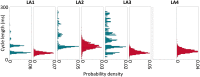
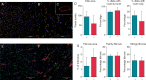
Methods and results: Arrhythmia wavefront dynamics were analysed for four optically mapped canine cholinergic AF preparations. A bilayer computer model was tuned to experimental preparations, and varied to have (i) fibrosis in both layers or the epicardium only, (ii) different spatial acetylcholine distributions, (iii) different intrinsic action potential duration between layers, and (iv) varied interlayer connectivity. Phase singularities (PSs) were identified and tracked over time to identify rotational drivers. New focal wavefronts were identified using phase contours. Phase singularity density and new wavefront locations were calculated during AF. There was a single dominant mechanism for sustaining AF in each of the preparations, either a rotational driver or repetitive new focal wavefronts. High-density PS sites existed preferentially around the pulmonary vein junctions. Three of the four preparations exhibited stable preferential sites of new wavefronts. Computational simulations predict that only a small number of connections are functionally important in sustaining AF, with new wavefront locations determined by the interplay between fibrosis distribution, acetylcholine concentration, and heterogeneity in repolarization within layers.
Conclusion: We were able to identify preferential sites of new wavefront initiation and rotational activity, in order to determine the mechanisms sustaining AF. Electrical measurements should be interpreted differently according to whether they are endocardial or epicardial recordings.
Figures





Similar articles
-
Endocardial-Epicardial Phase Mapping of Prolonged Persistent Atrial Fibrillation Recordings: High Prevalence of Dissociated Activation Patterns.Circ Arrhythm Electrophysiol. 2020 Aug;13(8):e008512. doi: 10.1161/CIRCEP.120.008512. Epub 2020 Jul 7. Circ Arrhythm Electrophysiol. 2020. PMID: 32634027
-
The effect of fixed and functional remodelling on conduction velocity, wavefront propagation, and rotational activity formation in atrial fibrillation.Europace. 2024 Oct 3;26(10):euae239. doi: 10.1093/europace/euae239. Europace. 2024. PMID: 39283961 Free PMC article.
-
Arrhythmogenic propensity of the fibrotic substrate after atrial fibrillation ablation: a longitudinal study using magnetic resonance imaging-based atrial models.Cardiovasc Res. 2019 Oct 1;115(12):1757-1765. doi: 10.1093/cvr/cvz083. Cardiovasc Res. 2019. PMID: 30977811 Free PMC article.
-
Pathophysiology of Paroxysmal and Persistent Atrial Fibrillation: Rotors, Foci and Fibrosis.Heart Lung Circ. 2017 Sep;26(9):887-893. doi: 10.1016/j.hlc.2017.05.119. Epub 2017 May 24. Heart Lung Circ. 2017. PMID: 28610723 Review.
-
Role of endo-epicardial dissociation of electrical activity and transmural conduction in the development of persistent atrial fibrillation.Prog Biophys Mol Biol. 2014 Aug;115(2-3):173-85. doi: 10.1016/j.pbiomolbio.2014.07.007. Epub 2014 Jul 30. Prog Biophys Mol Biol. 2014. PMID: 25086270 Review.
Cited by
-
Reconsidering the multiple wavelet hypothesis of atrial fibrillation.Heart Rhythm. 2020 Nov;17(11):1976-1983. doi: 10.1016/j.hrthm.2020.06.017. Epub 2020 Jun 22. Heart Rhythm. 2020. PMID: 32585192 Free PMC article.
-
Prevalence of endoepicardial asynchrony and breakthrough patterns in a bilayer computational model of heterogeneous endoepicardial dissociation in the left atrium.PLoS One. 2024 Nov 22;19(11):e0314342. doi: 10.1371/journal.pone.0314342. eCollection 2024. PLoS One. 2024. PMID: 39576793 Free PMC article.
-
Ganglionated Plexi Ablation for the Treatment of Atrial Fibrillation.J Clin Med. 2020 Sep 24;9(10):3081. doi: 10.3390/jcm9103081. J Clin Med. 2020. PMID: 32987820 Free PMC article. Review.
-
An Evaluation of Phase Analysis to Interpret Atrial Activation Patterns during Persistent Atrial Fibrillation for Targeted Ablation.J Clin Med. 2022 Sep 30;11(19):5807. doi: 10.3390/jcm11195807. J Clin Med. 2022. PMID: 36233675 Free PMC article.
-
Acetylcholine Delays Atrial Activation to Facilitate Atrial Fibrillation.Front Physiol. 2019 Sep 4;10:1105. doi: 10.3389/fphys.2019.01105. eCollection 2019. Front Physiol. 2019. PMID: 31551802 Free PMC article.
References
-
- Moe G, Rheinboldt W, Abildskov J.. A computer model of atrial fibrillation. Am Heart J 1964;67:200–20. - PubMed
-
- Verheule S, Tuyls E, van Hunnik A, Kuiper M, Schotten U, Allessie M.. Fibrillatory conduction in the atrial free walls of goats in persistent and permanent atrial fibrillation. Circ Arrhythm Electrophysiol 2010;3:590–9. - PubMed
-
- Lee S, Sahadevan J, Khrestian CM, Durand DM, Waldo AL.. High density mapping of atrial fibrillation during vagal nerve stimulation in the canine heart: restudying the Moe hypothesis. J Cardiovasc Electrophysiol 2013;24:328–35. - PubMed
-
- Sanders P, Berenfeld O, Hocini M, Jaïs P, Vaidyanathan R, Hsu L-F.. Spectral analysis identifies sites of high-frequency activity maintaining atrial fibrillation in humans. Circulation 2005;112:789–97. - PubMed

