Clustered DNA double-strand break formation and the repair pathway following heavy-ion irradiation
- PMID: 30476166
- PMCID: PMC6373698
- DOI: 10.1093/jrr/rry096
Clustered DNA double-strand break formation and the repair pathway following heavy-ion irradiation
Abstract
Photons, such as X- or γ-rays, induce DNA damage (distributed throughout the nucleus) as a result of low-density energy deposition. In contrast, particle irradiation with high linear energy transfer (LET) deposits high-density energy along the particle track. High-LET heavy-ion irradiation generates a greater number and more complex critical chromosomal aberrations, such as dicentrics and translocations, compared with X-ray or γ irradiation. In addition, the formation of >1000 bp deletions, which is rarely observed after X-ray irradiation, has been identified following high-LET heavy-ion irradiation. Previously, these chromosomal aberrations have been thought to be the result of misrepair of complex DNA lesions, defined as DNA damage through DNA double-strand breaks (DSBs) and single-strand breaks as well as base damage within 1-2 helical turns (<3-4 nm). However, because the scale of complex DNA lesions is less than a few nanometers, the large-scale chromosomal aberrations at a micrometer level cannot be simply explained by complex DNA lesions. Recently, we have demonstrated the existence of clustered DSBs along the particle track through the use of super-resolution microscopy. Furthermore, we have visualized high-level and frequent formation of DSBs at the chromosomal boundary following high-LET heavy-ion irradiation. In this review, we summarize the latest findings regarding the hallmarks of DNA damage structure and the repair pathway following heavy-ion irradiation. Furthermore, we discuss the mechanism through which high-LET heavy-ion irradiation may induce dicentrics, translocations and large deletions.
Figures


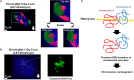
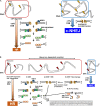
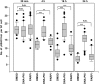

References
-
- Ritter S, Durante M. Heavy-ion induced chromosomal aberrations: a review. Mutat Res 2010;701:38–46. - PubMed
-
- Durante M, Cucinotta FA. Heavy ion carcinogenesis and human space exploration. Nat Rev Cancer 2008;8:465–72. - PubMed
-
- Durante M, Orecchia R, Loeffler JS. Charged-particle therapy in cancer: clinical uses and future perspectives. Nat Rev Clin Oncol 2017;14:483–95. - PubMed
-
- Veldeman L, Madani I, Hulstaert F et al. Evidence behind use of intensity-modulated radiotherapy: a systematic review of comparative clinical studies. Lancet Oncol 2008;9:367–75. - PubMed
-
- Loeffler JS, Durante M. Charged particle therapy—optimization, challenges and future directions. Nat Rev Clin Oncol 2013;10:411–24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

