A Chemiluminescent Probe for HNO Quantification and Real-Time Monitoring in Living Cells
- PMID: 30476360
- PMCID: PMC6396311
- DOI: 10.1002/anie.201811257
A Chemiluminescent Probe for HNO Quantification and Real-Time Monitoring in Living Cells
Abstract
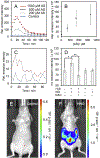
Azanone (HNO) is a reactive nitrogen species with pronounced biological activity and high therapeutic potential for cardiovascular dysfunction. A critical barrier to understanding the biology of HNO and furthering clinical development is the quantification and real-time monitoring of its delivery in living systems. Herein, we describe the design and synthesis of the first chemiluminescent probe for HNO, HNOCL-1, which can detect HNO generated from concentrations of Angeli's salt as low as 138 nm with high selectivity based on the reaction with a phosphine group to form a self-cleavable azaylide intermediate. We have capitalized on this high sensitivity to develop a generalizable kinetics-based approach, which provides real-time quantitative measurements of HNO concentration at the picomolar level. HNOCL-1 can monitor dynamics of HNO delivery in living cells and tissues, demonstrating the versatility of this method for tracking HNO in living systems.
Keywords: bioanalysis; chemiluminescence; nitroxyl; phosphine.
© 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

