Flavonoids from Nelumbo nucifera Gaertn., a Medicinal Plant: Uses in Traditional Medicine, Phytochemistry and Pharmacological Activities
- PMID: 30477094
- PMCID: PMC6313397
- DOI: 10.3390/medicines5040127
Flavonoids from Nelumbo nucifera Gaertn., a Medicinal Plant: Uses in Traditional Medicine, Phytochemistry and Pharmacological Activities
Abstract
Nelumbo nucifera Gaertn. has been used as an important ingredient for traditional medicines since ancient times, especially in Asian countries. Nowadays, many new or unknown phytochemical compounds from N. nucifera are still being discovered. Most of the current research about pharmacological activity focus on nuciferine, many other alkaloids, phenolic compounds, etc. However, there is no current review emphasizing on flavonoids, which is one of the potent secondary metabolites of this species and its pharmacological activities. Therefore, following a taxonomic description, we aim to illustrate and update the diversity of flavonoid phytochemical compounds from N. nucifera, the comparative analysis of flavonoid compositions and contents in various organs. The uses of this species in traditional medicine and the main pharmacological activities such as antioxidant, anti-inflammatory, anti-diabetic, anti-obesity, anti-angiogenic and anti-cancer activities are also illustrated in this works.
Keywords: Nelumbo nucifera; flavonoids; pharmacological activities; traditional medicine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


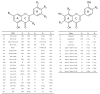

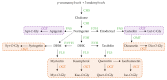
References
-
- Chen S., Zheng Y., Fang J.B., Liu Y.L., Li S.H. Flavonoids in lotus (Nelumbo) leaves evaluated by HPLC-MSnat the germplasm level. Food Res. Int. 2013;54:796–803. doi: 10.1016/j.foodres.2013.08.031. - DOI
-
- Sheikh S.A. Ethno-medicinal uses and pharmacological activities of lotus (Nelumbo nucifera) J. Med. Plants Stud. 2014;2:42–46.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

