Fatigue is Highly Prevalent in Patients with Asthma and Contributes to the Burden of Disease
- PMID: 30477110
- PMCID: PMC6306949
- DOI: 10.3390/jcm7120471
Fatigue is Highly Prevalent in Patients with Asthma and Contributes to the Burden of Disease
Abstract
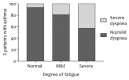
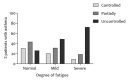
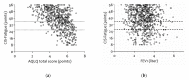
The 2018 update of the Global Strategy for Asthma Management and Prevention does not mention fatigue-related symptoms. Nevertheless, patients with asthma frequently report tiredness, lack of energy, and daytime sleepiness. Quantitative research regarding the prevalence of fatigue in asthmatic patients is lacking. This retrospective cross-sectional study of outpatients with asthma upon referral to a chest physician assessed fatigue (Checklist Individual Strength-Fatigue (CIS-Fatigue)), lung function (spirometry), asthma control (Asthma Control Questionnaire (ACQ)), dyspnea (Medical Research Council (MRC) scale), exercise capacity (six-minute walk test (6MWT)), and asthma-related Quality-of-Life (QoL), Asthma Quality of Life Questionnaire (AQLQ) during a comprehensive health-status assessment. In total, 733 asthmatic patients were eligible and analyzed (47.4 ± 16.3 years, 41.1% male). Severe fatigue (CIS-Fatigue ≥ 36 points) was detected in 62.6% of patients. Fatigue was not related to airflow limitation (FEV1, ρ = -0.083); was related moderately to ACQ (ρ = 0.455), AQLQ (ρ = -0.554), and MRC (ρ = 0.435; all p-values < 0.001); and was related weakly to 6MWT (ρ = -0.243, p < 0.001). In stepwise multiple regression analysis, 28.9% of variance in fatigue was explained by ACQ (21.0%), MRC (6.5%), and age (1.4%). As for AQLQ, 42.2% of variance was explained by fatigue (29.8%), MRC (8.6%), exacerbation rate (2.6%), and age (1.2%). Severe fatigue is highly prevalent in asthmatic patients; it is an important determinant of disease-specific QoL and a crucial yet ignored patient-related outcome in patients with asthma.
Keywords: asthma; fatigue; quality of life.
Conflict of interest statement
All authors (with the exception of M.A.S. and D.J.A.J.) have nothing to disclose. D.J.A.J. reports personal fees from Novartis, AstraZeneca, Boehringer Ingelheim, and GlaxoSmithKline outside the submitted work. M.A.S. reports grants from AstraZeneca, Boehringer Ingelheim, Netherlands Lung Foundation, and Stichting Astma Bestrijding during the conduct of the study; and personal fees from AstraZeneca, Boehringer Ingelheim, Novartis, Teva, and GlaxoSmithKline outside the submitted work.
Figures

References
-
- Global Initiative for Asthma Global Strategy for Asthma Management and Prevention. [(accessed on 7 July 2018)];2018 Available online: www.ginasthma.org.
-
- Svedsater H., Roberts J., Patel C., Macey J., Hilton E., Bradshaw L. Life Impact and Treatment Preferences of Individuals with Asthma and Chronic Obstructive Pulmonary Disease: Results from Qualitative Interviews and Focus Groups. Adv. Ther. 2017;34:1466–1481. doi: 10.1007/s12325-017-0557-0. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

