Elicited ROS Scavenging Activity, Photoprotective, and Wound-Healing Properties of Collagen-Derived Peptides from the Marine Sponge Chondrosia reniformis
- PMID: 30477144
- PMCID: PMC6316299
- DOI: 10.3390/md16120465
Elicited ROS Scavenging Activity, Photoprotective, and Wound-Healing Properties of Collagen-Derived Peptides from the Marine Sponge Chondrosia reniformis
Abstract
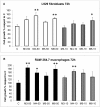
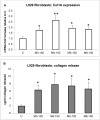
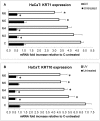
Recently, the bioactive properties of marine collagen and marine collagen hydrolysates have been demonstrated. Although there is some literature assessing the general chemical features and biocompatibility of collagen extracts from marine sponges, no data are available on the biological effects of sponge collagen hydrolysates for biomedical and/or cosmetic purposes. Here, we studied the in vitro toxicity, antioxidant, wound-healing, and photoprotective properties of four HPLC-purified fractions of trypsin-digested collagen extracts-marine collagen hydrolysates (MCHs)-from the marine sponge C. reniformis. The results showed that the four MCHs have no degree of toxicity on the cell lines analyzed; conversely, they were able to stimulate cell growth. They showed a significant antioxidant activity both in cell-free assays as well as in H₂O₂ or quartz-stimulated macrophages, going from 23% to 60% of reactive oxygen species (ROS) scavenging activity for the four MCHs. Finally, an in vitro wound-healing test was performed with fibroblasts and keratinocytes, and the survival of both cells was evaluated after UV radiation. In both experiments, MCHs showed significant results, increasing the proliferation speed and protecting from UV-induced cell death. Overall, these data open the way to the use of C. reniformis MCHs in drug and cosmetic formulations for damaged or photoaged skin repair.
Keywords: antioxidant; collagen hydrolysates; cosmetics; inflammation; marine collagen peptide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









Similar articles
-
Anti-photoaging and photoprotective compounds derived from marine organisms.Mar Drugs. 2010 Apr 8;8(4):1189-202. doi: 10.3390/md8041189. Mar Drugs. 2010. PMID: 20479974 Free PMC article. Review.
-
On the Path to Thermo-Stable Collagen: Culturing the Versatile Sponge Chondrosia reniformis.Mar Drugs. 2021 Nov 26;19(12):669. doi: 10.3390/md19120669. Mar Drugs. 2021. PMID: 34940668 Free PMC article.
-
Production, Characterization and Biocompatibility Evaluation of Collagen Membranes Derived from Marine Sponge Chondrosia reniformis Nardo, 1847.Mar Drugs. 2018 Mar 29;16(4):111. doi: 10.3390/md16040111. Mar Drugs. 2018. PMID: 29596370 Free PMC article.
-
Pichia pastoris production of a prolyl 4-hydroxylase derived from Chondrosia reniformis sponge: A new biotechnological tool for the recombinant production of marine collagen.J Biotechnol. 2015 Aug 20;208:28-36. doi: 10.1016/j.jbiotec.2015.05.007. Epub 2015 May 27. J Biotechnol. 2015. PMID: 26022422
-
Marine Collagen: A Promising Biomaterial for Wound Healing, Skin Anti-Aging, and Bone Regeneration.Mar Drugs. 2022 Jan 10;20(1):61. doi: 10.3390/md20010061. Mar Drugs. 2022. PMID: 35049916 Free PMC article. Review.
Cited by
-
Spirulina protein promotes skin wound repair in a mouse model of full-thickness dermal excisional wound.Int J Mol Med. 2020 Jul;46(1):351-359. doi: 10.3892/ijmm.2020.4571. Epub 2020 Apr 8. Int J Mol Med. 2020. PMID: 32319537 Free PMC article.
-
Potential Biomedical Applications of Collagen Filaments derived from the Marine Demosponges Ircinia oros (Schmidt, 1864) and Sarcotragus foetidus (Schmidt, 1862).Mar Drugs. 2021 Oct 6;19(10):563. doi: 10.3390/md19100563. Mar Drugs. 2021. PMID: 34677462 Free PMC article.
-
Marine cosmetics and the blue bioeconomy: From sourcing to success stories.iScience. 2024 Nov 6;27(12):111339. doi: 10.1016/j.isci.2024.111339. eCollection 2024 Dec 20. iScience. 2024. PMID: 39650733 Free PMC article. Review.
-
The Characterization and Cytotoxic Evaluation of Chondrosia reniformis Collagen Isolated from Different Body Parts (Ectosome and Choanosome) Envisaging the Development of Biomaterials.Mar Drugs. 2024 Jan 24;22(2):55. doi: 10.3390/md22020055. Mar Drugs. 2024. PMID: 38393026 Free PMC article.
-
Abalone Collagen Extracts Potentiate Stem Cell Properties of Human Epidermal Keratinocytes.Mar Drugs. 2019 Jul 20;17(7):424. doi: 10.3390/md17070424. Mar Drugs. 2019. PMID: 31330853 Free PMC article.
References
-
- Meena C., Mengi S., Deshpande S. Biomedical and industrial applications of collagen. J. Chem. Sci. 1999;111:319–329.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

