Screening of a Library of Oligosaccharides Targeting Lectin LecB of Pseudomonas Aeruginosa and Synthesis of High Affinity Oligoglycoclusters
- PMID: 30477231
- PMCID: PMC6321166
- DOI: 10.3390/molecules23123073
Screening of a Library of Oligosaccharides Targeting Lectin LecB of Pseudomonas Aeruginosa and Synthesis of High Affinity Oligoglycoclusters
Abstract
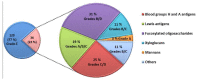
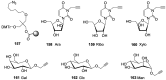
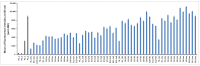
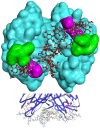
The Gram negative bacterium Pseudomonas aeruginosa (PA) is an opportunistic bacterium that causes severe and chronic infection of immune-depressed patients. It has the ability to form a biofilm that gives a selective advantage to the bacteria with respect to antibiotherapy and host defenses. Herein, we have focused on the tetrameric soluble lectin which is involved in bacterium adherence to host cells, biofilm formation, and cytotoxicity. It binds to l-fucose, d-mannose and glycan exposing terminal fucose or mannose. Using a competitive assay on microarray, 156 oligosaccharides and polysaccharides issued from fermentation or from the biomass were screened toward their affinity to LecB. Next, the five best ligands (Lewisa, Lewisb, Lewisx, siayl-Lewisx and 3-fucosyllactose) were derivatized with a propargyl aglycon allowing the synthesis of 25 trivalent, 25 tetravalent and 5 monovalent constructions thanks to copper catalyzed azide alkyne cycloaddition. The 55 clusters were immobilized by DNA Directed immobilization leading to the fabrication of a glycocluster microarray. Their binding to LecB was studied. Multivalency improved the binding to LecB. The binding structure relationship of the clusters is mainly influenced by the carbohydrate residues. Molecular simulations indicated that the simultaneous contact of both binding sites of monomer A and D seems to be energetically possible.
Keywords: Pseudomonas aeruginosa; anti-bacterial; glycocluster; glycoconjugate; oligosaccharide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









Similar articles
-
Importance of topology for glycocluster binding to Pseudomonas aeruginosa and Burkholderia ambifaria bacterial lectins.Org Biomol Chem. 2015 Dec 14;13(46):11244-54. doi: 10.1039/c5ob01445j. Epub 2015 Sep 28. Org Biomol Chem. 2015. PMID: 26412676
-
Synthesis of mannoheptose derivatives and their evaluation as inhibitors of the lectin LecB from the opportunistic pathogen Pseudomonas aeruginosa.Carbohydr Res. 2015 Aug 14;412:34-42. doi: 10.1016/j.carres.2015.04.010. Epub 2015 May 5. Carbohydr Res. 2015. PMID: 26004349
-
Glycopeptide dendrimers with high affinity for the fucose-binding lectin LecB from Pseudomonas aeruginosa.ChemMedChem. 2009 Apr;4(4):562-9. doi: 10.1002/cmdc.200800380. ChemMedChem. 2009. PMID: 19189366
-
Glycopeptide dendrimers as Pseudomonas aeruginosa biofilm inhibitors.Chem Soc Rev. 2013 Jun 7;42(11):4814-22. doi: 10.1039/c3cs35504g. Epub 2013 Feb 1. Chem Soc Rev. 2013. PMID: 23370573 Review.
-
Structural Considerations for Building Synthetic Glycoconjugates as Inhibitors for Pseudomonas aeruginosa Lectins.ChemMedChem. 2022 Jun 20;17(12):e202200081. doi: 10.1002/cmdc.202200081. Epub 2022 May 3. ChemMedChem. 2022. PMID: 35426976 Free PMC article. Review.
Cited by
-
Dismantling the bacterial glycocalyx: Chemical tools to probe, perturb, and image bacterial glycans.Bioorg Med Chem. 2021 Jul 15;42:116268. doi: 10.1016/j.bmc.2021.116268. Epub 2021 Jun 7. Bioorg Med Chem. 2021. PMID: 34130219 Free PMC article. Review.
-
The Pseudomonas aeruginosa Lectin LecB Causes Integrin Internalization and Inhibits Epithelial Wound Healing.mBio. 2020 Mar 10;11(2):e03260-19. doi: 10.1128/mBio.03260-19. mBio. 2020. PMID: 32156827 Free PMC article.
References
-
- Sommer R., Wagner S., Rox K., Varrot A., Hauck D., Wamhoff E.C., Schreiber J., Ryckmans T., Brunner T., Rademacher C., et al. Glycomimetic, Orally Bioavailable LecB Inhibitors Block Biofilm Formation of Pseudomonas aeruginosa. J. Am. Chem. Soc. 2018;140:2537–2545. doi: 10.1021/jacs.7b11133. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

