Autologous Gene and Cell Therapy Provides Safe and Long-Term Curative Therapy in A Large Pig Model of Hereditary Tyrosinemia Type 1
- PMID: 30477316
- PMCID: PMC6322137
- DOI: 10.1177/0963689718814188
Autologous Gene and Cell Therapy Provides Safe and Long-Term Curative Therapy in A Large Pig Model of Hereditary Tyrosinemia Type 1
Abstract
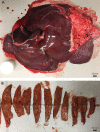
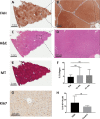
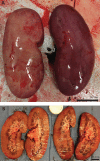
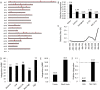
Orthotopic liver transplantation remains the only curative therapy for inborn errors of metabolism. Given the tremendous success for primary immunodeficiencies using ex-vivo gene therapy with lentiviral vectors, there is great interest in developing similar curative therapies for metabolic liver diseases. We have previously generated a pig model of hereditary tyrosinemia type 1 (HT1), an autosomal recessive disorder caused by deficiency of fumarylacetoacetate hydrolase (FAH). Using this model, we have demonstrated curative ex-vivo gene and cell therapy using a lentiviral vector to express FAH in autologous hepatocytes. To further evaluate the long-term clinical outcomes of this therapeutic approach, we continued to monitor one of these pigs over the course of three years. The animal continued to thrive off the protective drug NTBC, gaining weight appropriately, and maintaining sexual fecundity for the course of his life. The animal was euthanized 31 months after transplantation to perform a thorough biochemical and histological analysis. Biochemically, liver enzymes and alpha-fetoprotein levels remained normal and abhorrent metabolites specific to HT1 remained corrected. Liver histology showed no evidence of tumorigenicity and Masson's trichrome staining revealed minimal fibrosis and no evidence of cirrhosis. FAH-immunohistochemistry revealed complete repopulation of the liver by transplanted FAH-positive cells. A complete histopathological report on other organs, including kidney, revealed no abnormalities. This study is the first to demonstrate long-term safety and efficacy of hepatocyte-directed gene therapy in a large animal model. We conclude that hepatocyte-directed ex-vivo gene therapy is a rational choice for further exploration as an alternative therapeutic approach to whole organ transplantation for metabolic liver disease, including HT1.
Keywords: gene therapy; fumarylacetoacetate hydrolase; hepatocyte transplantation; hereditary tyrosinemia type 1; porcine model.
Conflict of interest statement
Figures


Similar articles
-
Curative ex vivo liver-directed gene therapy in a pig model of hereditary tyrosinemia type 1.Sci Transl Med. 2016 Jul 27;8(349):349ra99. doi: 10.1126/scitranslmed.aaf3838. Sci Transl Med. 2016. PMID: 27464750 Free PMC article.
-
Curative Ex Vivo Hepatocyte-Directed Gene Editing in a Mouse Model of Hereditary Tyrosinemia Type 1.Hum Gene Ther. 2018 Nov;29(11):1315-1326. doi: 10.1089/hum.2017.252. Epub 2018 Jun 22. Hum Gene Ther. 2018. PMID: 29764210 Free PMC article.
-
Fumarylacetoacetate Hydrolase Knock-out Rabbit Model for Hereditary Tyrosinemia Type 1.J Biol Chem. 2017 Mar 17;292(11):4755-4763. doi: 10.1074/jbc.M116.764787. Epub 2017 Jan 4. J Biol Chem. 2017. PMID: 28053091 Free PMC article.
-
Biochemical and Clinical Aspects of Hereditary Tyrosinemia Type 1.Adv Exp Med Biol. 2017;959:9-21. doi: 10.1007/978-3-319-55780-9_2. Adv Exp Med Biol. 2017. PMID: 28755181 Review.
-
The Liver in Tyrosinemia Type I: Clinical Management and Course in Quebec.Adv Exp Med Biol. 2017;959:75-83. doi: 10.1007/978-3-319-55780-9_6. Adv Exp Med Biol. 2017. PMID: 28755185 Review.
Cited by
-
Development of RAG2 -/- IL2Rγ -/Y immune deficient FAH-knockout miniature pig.Front Immunol. 2022 Aug 9;13:950194. doi: 10.3389/fimmu.2022.950194. eCollection 2022. Front Immunol. 2022. PMID: 36032112 Free PMC article.
-
Novel vectors and approaches for gene therapy in liver diseases.JHEP Rep. 2021 Apr 30;3(4):100300. doi: 10.1016/j.jhepr.2021.100300. eCollection 2021 Aug. JHEP Rep. 2021. PMID: 34159305 Free PMC article. Review.
-
The future of gene-targeted therapy for hereditary tyrosinemia type 1 as a lead indication among the inborn errors of metabolism.Expert Opin Orphan Drugs. 2020;8(7):245-256. doi: 10.1080/21678707.2020.1791082. Epub 2020 Jul 21. Expert Opin Orphan Drugs. 2020. PMID: 33224636 Free PMC article.
-
Gene Therapy for Inherited Liver Disease: To Add or to Edit.Int J Mol Sci. 2024 Nov 21;25(23):12514. doi: 10.3390/ijms252312514. Int J Mol Sci. 2024. PMID: 39684224 Free PMC article. Review.
-
Direct conversion of porcine primary fibroblasts into hepatocyte-like cells.Sci Rep. 2021 Apr 29;11(1):9334. doi: 10.1038/s41598-021-88727-1. Sci Rep. 2021. PMID: 33927320 Free PMC article.
References
-
- Grompe M. The pathophysiology and treatment of hereditary tyrosinemia type 1. Semin Liver Dis. 2001;21(4):563–571. - PubMed
-
- Malik S, Kassai B, Cochat P. Overview of pediatric organ transplantation: current opinion and future perspectives on immunosuppression. Curr Opin Organ Transplant. 2015;20(5):527–535. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

