The relevance of cerebrospinal fluid α-synuclein levels to sporadic and familial Alzheimer's disease
- PMID: 30477568
- PMCID: PMC6260771
- DOI: 10.1186/s40478-018-0624-z
The relevance of cerebrospinal fluid α-synuclein levels to sporadic and familial Alzheimer's disease
Abstract
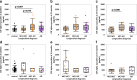
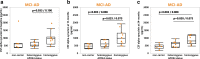
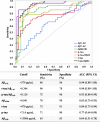
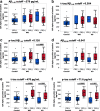
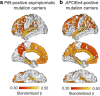
Accumulating evidence demonstrating higher cerebrospinal fluid (CSF) α-synuclein (αSyn) levels and αSyn pathology in the brains of Alzheimer's disease (AD) patients suggests that αSyn is involved in the pathophysiology of AD. To investigate whether αSyn could be related to specific aspects of the pathophysiology present in both sporadic and familial disease, we quantified CSF levels of αSyn and assessed links to various disease parameters in a longitudinally followed cohort (n = 136) including patients with sporadic mild cognitive impairment (MCI) and AD, and in a cross-sectional sample from the Dominantly Inherited Alzheimer's Network (n = 142) including participants carrying autosomal dominant AD (ADAD) gene mutations and their non-mutation carrying family members.Our results show that sporadic MCI patients that developed AD over a period of two years exhibited higher baseline αSyn levels (p = 0.03), which inversely correlated to their Mini-Mental State Examination scores, compared to cognitively normal controls (p = 0.02). In the same patients, there was a dose-dependent positive association between CSF αSyn and the APOEε4 allele. Further, CSF αSyn levels were higher in symptomatic ADAD mutation carriers versus non-mutation carriers (p = 0.03), and positively correlated to the estimated years from symptom onset (p = 0.05) across all mutation carriers. In asymptomatic (Clinical Dementia Rating < 0.5) PET amyloid-positive ADAD mutation carriers CSF αSyn was positively correlated to 11C-Pittsburgh Compound-B (PiB) retention in several brain regions including the posterior cingulate, superior temporal and frontal cortical areas. Importantly, APOEε4-positive ADAD mutation carriers exhibited an association between CSF αSyn levels and mean cortical PiB retention (p = 0.032). In both the sporadic AD and ADAD cohorts we found several associations predominantly between CSF levels of αSyn, tau and amyloid-β1-40.Our results suggest that higher CSF αSyn levels are linked to AD pathophysiology at the early stages of disease development and to the onset of cognitive symptoms in both sporadic and autosomal dominant AD. We conclude that APOEε4 may promote the processes driven by αSyn, which in turn may reflect on molecular mechanisms linked to the asymptomatic build-up of amyloid plaque burden in brain regions involved in the early stages of AD development.
Keywords: APOEε4; Alzheimer’s disease; Biomarkers; Mild cognitive impairment; alpha-synuclein.
Conflict of interest statement
Ethics approval and consent to participate
Research aims pursued in the longitudinal cohort were approved by the regional ethics committee in Trondheim, Norway (2010/226) and Stockholm, Sweden (2016/771–31/4). The described studies relating to the DIAN cohort wereapproved by the local ethics committee in Stockholm, Sweden (2016/2114–31/4). The studies of both cohorts were carried out in agreement with the Helsinki Declaration.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
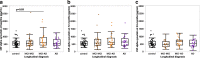




References
-
- Berge G, Lauridsen C, Sando SB, Holder DJ, Møller I, Aasly JO, et al. Effect of Tween-20 on Core biomarkers measured in cerebrospinal fluid from patients with Alzheimer’s disease, mild cognitive impairment, or healthy control individuals. J Alzheimer’s Dis. 2015;49:493–502. doi: 10.3233/JAD-150234. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

