Dual functions for OVAAL in initiation of RAF/MEK/ERK prosurvival signals and evasion of p27-mediated cellular senescence
- PMID: 30478051
- PMCID: PMC6294934
- DOI: 10.1073/pnas.1805950115
Dual functions for OVAAL in initiation of RAF/MEK/ERK prosurvival signals and evasion of p27-mediated cellular senescence
Erratum in
-
Correction for Sang et al., Dual functions for OVAAL in initiation of RAF/MEK/ERK prosurvival signals and evasion of p27-mediated cellular senescence.Proc Natl Acad Sci U S A. 2021 Sep 28;118(39):e2114831118. doi: 10.1073/pnas.2114831118. Proc Natl Acad Sci U S A. 2021. PMID: 34544884 Free PMC article. No abstract available.
Abstract
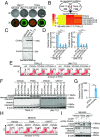
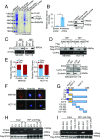
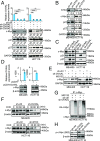
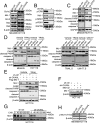
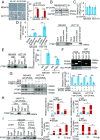
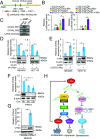
Long noncoding RNAs (lncRNAs) function through a diverse array of mechanisms that are not presently fully understood. Here, we sought to find lncRNAs differentially regulated in cancer cells resistant to either TNF-related apoptosis-inducing ligand (TRAIL) or the Mcl-1 inhibitor UMI-77, agents that act through the extrinsic and intrinsic apoptotic pathways, respectively. This work identified a commonly up-regulated lncRNA, ovarian adenocarcinoma-amplified lncRNA (OVAAL), that conferred apoptotic resistance in multiple cancer types. Analysis of clinical samples revealed OVAAL expression was significantly increased in colorectal cancers and melanoma in comparison to the corresponding normal tissues. Functional investigations showed that OVAAL depletion significantly inhibited cancer cell proliferation and retarded tumor xenograft growth. Mechanically, OVAAL physically interacted with serine/threonine-protein kinase 3 (STK3), which, in turn, enhanced the binding between STK3 and Raf-1. The ternary complex OVAAL/STK3/Raf-1 enhanced the activation of the RAF protooncogene serine/threonine-protein kinase (RAF)/mitogen-activated protein kinase kinase 1 (MEK)/ERK signaling cascade, thus promoting c-Myc-mediated cell proliferation and Mcl-1-mediated cell survival. On the other hand, depletion of OVAAL triggered cellular senescence through polypyrimidine tract-binding protein 1 (PTBP1)-mediated p27 expression, which was regulated by competitive binding between OVAAL and p27 mRNA to PTBP1. Additionally, c-Myc was demonstrated to drive OVAAL transcription, indicating a positive feedback loop between c-Myc and OVAAL in controlling tumor growth. Taken together, these results reveal that OVAAL contributes to the survival of cancer cells through dual mechanisms controlling RAF/MEK/ERK signaling and p27-mediated cell senescence.
Keywords: OVAAL; c-Myc; p27; proliferation; senescence.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Opposite long-term regulation of c-Myc and p27Kip1 through overactivation of Raf-1 and the MEK/ERK module in proliferating human choroidal melanoma cells.Oncogene. 2003 Dec 4;22(55):8813-22. doi: 10.1038/sj.onc.1207099. Oncogene. 2003. PMID: 14654778
-
Complementation of defective colony-stimulating factor 1 receptor signaling and mitogenesis by Raf and v-Src.Mol Cell Biol. 1999 Feb;19(2):1101-15. doi: 10.1128/MCB.19.2.1101. Mol Cell Biol. 1999. PMID: 9891045 Free PMC article.
-
Different effects of point mutations within the B-Raf glycine-rich loop in colorectal tumors on mitogen-activated protein/extracellular signal-regulated kinase kinase/extracellular signal-regulated kinase and nuclear factor kappaB pathway and cellular transformation.Cancer Res. 2004 May 15;64(10):3428-35. doi: 10.1158/0008-5472.CAN-03-3591. Cancer Res. 2004. PMID: 15150094
-
Epigenetic regulation in human melanoma: past and future.Epigenetics. 2015;10(2):103-21. doi: 10.1080/15592294.2014.1003746. Epigenetics. 2015. PMID: 25587943 Free PMC article. Review.
-
The Raf/MEK/ERK pathway: new concepts of activation.Biol Cell. 2001 Sep;93(1-2):53-62. doi: 10.1016/s0248-4900(01)01125-x. Biol Cell. 2001. PMID: 11730323 Review.
Cited by
-
Molecular mechanisms of alveolar epithelial cell senescence and idiopathic pulmonary fibrosis: a narrative review.J Thorac Dis. 2023 Jan 31;15(1):186-203. doi: 10.21037/jtd-22-886. Epub 2022 Dec 27. J Thorac Dis. 2023. PMID: 36794134 Free PMC article. Review.
-
MEF2A-mediated lncRNA HCP5 Inhibits Gastric Cancer Progression via MiR-106b-5p/p21 Axis.Int J Biol Sci. 2021 Jan 16;17(2):623-634. doi: 10.7150/ijbs.55020. eCollection 2021. Int J Biol Sci. 2021. PMID: 33613117 Free PMC article.
-
lncRNA-Triggered Macrophage Inflammaging Deteriorates Age-Related Diseases.Mediators Inflamm. 2019 Dec 21;2019:4260309. doi: 10.1155/2019/4260309. eCollection 2019. Mediators Inflamm. 2019. PMID: 31949425 Free PMC article. Review.
-
NEAT1 Overexpression Indicates a Poor Prognosis and Induces Chemotherapy Resistance via the miR-491-5p/SOX3 Signaling Pathway in Ovarian Cancer.Front Genet. 2021 Apr 29;12:616220. doi: 10.3389/fgene.2021.616220. eCollection 2021. Front Genet. 2021. Retraction in: Front Genet. 2022 Jan 31;13:861109. doi: 10.3389/fgene.2022.861109. PMID: 33995475 Free PMC article. Retracted.
-
SENEBLOC, a long non-coding RNA suppresses senescence via p53-dependent and independent mechanisms.Nucleic Acids Res. 2020 Apr 6;48(6):3089-3102. doi: 10.1093/nar/gkaa063. Nucleic Acids Res. 2020. PMID: 32030426 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

