Multidirectional desymmetrization of pluripotent building block en route to diastereoselective synthesis of complex nature-inspired scaffolds
- PMID: 30478283
- PMCID: PMC6255838
- DOI: 10.1038/s41467-018-07521-2
Multidirectional desymmetrization of pluripotent building block en route to diastereoselective synthesis of complex nature-inspired scaffolds
Abstract
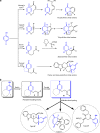
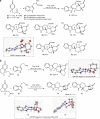
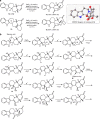
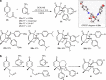
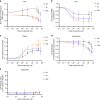
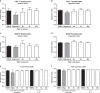
Octahydroindolo[2,3-a]quinolizine ring system forms the basic framework comprised of more than 2000 distinct family members of natural products. Despite the potential applications of this privileged substructure in drug discovery, efficient, atom-economic and modular strategies for its assembly, is underdeveloped. Here we show a one-step build/couple/pair strategy that uniquely allows access to diverse octahydroindolo[2,3-a]quinolizine scaffolds with more than three contiguous chiral centers and broad distribution of molecular shapes via desymmetrization of the oxidative-dearomatization products of phenols. The cascade demonstrates excellent diastereoselectivity, and the enantioselectivity exceeded 99% when amino acids are used as chiral reagents. Furthermore, two diastereoselective reactions for the synthesis of oxocanes and piperazinones, is reported. Phenotypic screening of the octahydroindolo[2,3-a]quinolizine library identifies small molecule probes that selectively suppress mitochondrial membrane potential, ATP contents and elevate the ROS contents in hepatoma cells (Hepa1-6) without altering the immunological activation or reprogramming of T- and B-cells, a promising approach to cancer therapy.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

