Revisiting the initial steps of sexual development in the malaria parasite Plasmodium falciparum
- PMID: 30478286
- PMCID: PMC6294672
- DOI: 10.1038/s41564-018-0291-7
Revisiting the initial steps of sexual development in the malaria parasite Plasmodium falciparum
Abstract
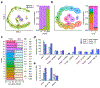
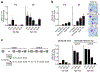
Human to vector transmission of malaria requires that some blood-stage parasites abandon asexual growth and convert into non-replicating sexual forms called gametocytes. The initial steps of gametocytogenesis remain largely uncharacterized. Here, we study this part of the malaria life cycle in Plasmodium falciparum using PfAP2-G, the master regulator of sexual conversion, as a marker of commitment. We demonstrate the existence of PfAP2-G-positive sexually committed parasite stages that precede the previously known committed schizont stage. We also found that sexual conversion can occur by two different routes: the previously described route in which PfAP2-G-expressing parasites complete a replicative cycle as committed forms before converting into gametocytes upon re-invasion, or a direct route with conversion within the same cycle as initial PfAP2-G expression. The latter route is linked to early PfAP2-G expression in ring stages. Reanalysis of published single-cell RNA-sequencing (RNA-seq) data confirmed the presence of both routes. Consistent with these results, using plaque assays we observed that, in contrast to the prevailing model, many schizonts produced mixed plaques containing both asexual parasites and gametocytes. Altogether, our results reveal unexpected features of the initial steps of sexual development and extend the current view of this part of the malaria life cycle.
Conflict of interest statement
COMPETING INTERESTS STATEMENT
The authors declare no competing interests.
Figures



Comment in
-
Commitment Isn't for Everyone.Trends Parasitol. 2019 Jun;35(6):381-383. doi: 10.1016/j.pt.2019.03.012. Epub 2019 Apr 30. Trends Parasitol. 2019. PMID: 31053335 Free PMC article.
References
REFERENCES THAT APPEAR ONLY IN THE METHODS
-
- Cortés A, Benet A, Cooke BM, Barnwell JW & Reeder JC Ability of Plasmodium falciparum to invade Southeast Asian ovalocytes varies between parasite lines. Blood 104, 2961–2966 (2004). - PubMed
-
- Delves MJ et al. Routine in vitro culture of P. falciparum gametocytes to evaluate novel transmission-blocking interventions. Nat Protoc 11, 1668–1680 (2016). - PubMed

