An upstream protein-coding region in enteroviruses modulates virus infection in gut epithelial cells
- PMID: 30478287
- PMCID: PMC6443042
- DOI: 10.1038/s41564-018-0297-1
An upstream protein-coding region in enteroviruses modulates virus infection in gut epithelial cells
Abstract
Enteroviruses comprise a large group of mammalian pathogens that includes poliovirus. Pathology in humans ranges from sub-clinical to acute flaccid paralysis, myocarditis and meningitis. Until now, all of the enteroviral proteins were thought to derive from the proteolytic processing of a polyprotein encoded in a single open reading frame. Here we report that many enterovirus genomes also harbour an upstream open reading frame (uORF) that is subject to strong purifying selection. Using echovirus 7 and poliovirus 1, we confirmed the expression of uORF protein in infected cells. Through ribosome profiling (a technique for the global footprinting of translating ribosomes), we also demonstrated translation of the uORF in representative members of the predominant human enterovirus species, namely Enterovirus A, B and C. In differentiated human intestinal organoids, uORF protein-knockout echoviruses are attenuated compared to the wild-type at late stages of infection where membrane-associated uORF protein facilitates virus release. Thus, we have identified a previously unknown enterovirus protein that facilitates virus growth in gut epithelial cells-the site of initial viral invasion into susceptible hosts. These findings overturn the 50-year-old dogma that enteroviruses use a single-polyprotein gene expression strategy and have important implications for the understanding of enterovirus pathogenesis.
Conflict of interest statement
The authors declare no competing interests.
Figures


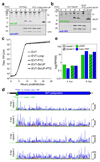


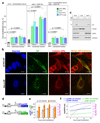
References
-
- Bedard KM, Semler BL. Regulation of picornavirus gene expression. Microbes Infect. 2004;6:702–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

