High glycolytic activity of tumor cells leads to underestimation of electron transport system capacity when mitochondrial ATP synthase is inhibited
- PMID: 30478338
- PMCID: PMC6255871
- DOI: 10.1038/s41598-018-35679-8
High glycolytic activity of tumor cells leads to underestimation of electron transport system capacity when mitochondrial ATP synthase is inhibited
Abstract
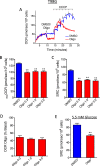
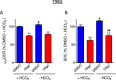
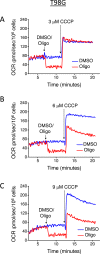
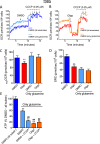
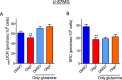
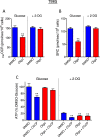
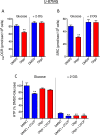
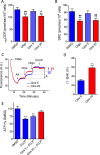
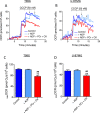
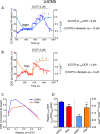
This study sought to elucidate how oligomycin, an ATP synthase blocker, leads to underestimation of maximal oxygen consumption rate (maxOCR) and spare respiratory capacity (SRC) in tumor cells. T98G and U-87MG glioma cells were titrated with the protonophore CCCP to induce maxOCR. The presence of oligomycin (0.3-3.0 µg/mL) led to underestimation of maxOCR and a consequent decrease in SRC values of between 25% and 40% in medium containing 5.5 or 11 mM glucose. The inhibitory effect of oligomycin on CCCP-induced maxOCR did not occur when glutamine was the metabolic substrate or when the glycolytic inhibitor 2-deoxyglucose was present. ATP levels were reduced and ADP/ATP ratios increased in cells treated with CCCP, but these changes were minimized when oligomycin was used to inhibit reverse activity of ATP synthase. Exposing digitonin-permeabilized cells to exogenous ATP, but not ADP, resulted in partial inhibition of CCCP-induced maxOCR. We conclude that underestimation of maxOCR and SRC in tumor cells when ATP synthase is inhibited is associated with high glycolytic activity and that the glycolytic ATP yield may have an inhibitory effect on the metabolism of respiratory substrates and cytochrome c oxidase activity. Under CCCP-induced maxOCR, oligomycin preserves intracellular ATP by inhibiting ATP synthase reverse activity.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

