Time-resolved fluorescence measurements on leaves: principles and recent developments
- PMID: 30478711
- PMCID: PMC6509100
- DOI: 10.1007/s11120-018-0607-8
Time-resolved fluorescence measurements on leaves: principles and recent developments
Abstract
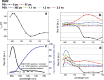
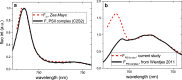
Photosynthesis starts when a pigment in the photosynthetic antennae absorbs a photon. The electronic excitation energy is then transferred through the network of light-harvesting pigments to special chlorophyll (Chl) molecules in the reaction centres, where electron transfer is initiated. Energy transfer and primary electron transfer processes take place on timescales ranging from femtoseconds to nanoseconds, and can be monitored in real time via time-resolved fluorescence spectroscopy. This method is widely used for measurements on unicellular photosynthetic organisms, isolated photosynthetic membranes, and individual complexes. Measurements on intact leaves remain a challenge due to their high structural heterogeneity, high scattering, and high optical density, which can lead to optical artefacts. However, detailed information on the dynamics of these early steps, and the underlying structure-function relationships, is highly informative and urgently required in order to get deeper insights into the physiological regulation mechanisms of primary photosynthesis. Here, we describe a current methodology of time-resolved fluorescence measurements on intact leaves in the picosecond to nanosecond time range. Principles of fluorescence measurements on intact leaves, possible sources of alterations of fluorescence kinetics and the ways to overcome them are addressed. We also describe how our understanding of the organisation and function of photosynthetic proteins and energy flow dynamics in intact leaves can be enriched through the application of time-resolved fluorescence spectroscopy on leaves. For that, an example of a measurement on Zea mays leaves is presented.
Keywords: Fluorescence; Leaf; Re-absorption; Time-resolved spectroscopy.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- Agati G, Fusi F, Mazzinghi P, Dipaolo ML. A simple approach to the evaluation of the reabsorption of chlorophyll fluorescence-spectra in intact leaves. J Photochem Photobiol B. 1993;17(2):163–171.
-
- Ahmad P, Prasad MNV. Environmental adaptations and stress tolerance of plants in the era of climate change. New York: Springer; 2012.
-
- Akimoto S, Yokono M, Hamada F, Teshigahara A, Aikawa S, Kondo A. Adaptation of light-harvesting systems of Arthrospira platensis to light conditions, probed by time-resolved fluorescence spectroscopy. Biochim Biophys Acta. 2012;1817(8):1483–1489. - PubMed
-
- Andrizhiyevskaya EG, Chojnicka A, Bautista JA, Diner BA, van Grondelle R, Dekker JP. Origin of the F685 and F695 fluorescence in photosystem II. Photosynth Res. 2005;84(1–3):173–180. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

