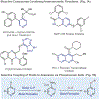
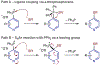
Selective formation of heteroaryl thioethers via a phosphonium ion coupling reaction
- PMID: 30479455
- PMCID: PMC6252084
- DOI: 10.1016/j.tet.2017.12.040
Selective formation of heteroaryl thioethers via a phosphonium ion coupling reaction
Abstract
Heteroaryl thioethers, comprised of pyridines and diazines, are an important class of compounds with relevance to medicinal chemistry. Metal-catalyzed cross-couplings and SNAr are traditionally used to form C-S bonds in these systems but are limited by available halogenated precursors. An alternative approach is presented where pyridines and diazines are transformed into heterocyclic phosphonium salts and then C-S bonds are formed by adding thiolate nucleophiles. The process is 4-selective for pyridines, simple to execute and can be used to make derivatives of complex pharmaceuticals.
Keywords: C-S Bonds; Diazines; Heteroaryl thioethers; Late-stage functionalization; Phosphonium salts; Pyridines.
Figures
References
-
- Feng M; Tang B; Liang S; Jiang X Curr. Top. Med. Chem 2016, 16, 1200; - PMC - PubMed
- Ilardi EA; Vitaku E; Njardarson JT; J. Med. Chem 2014, 57, 2832; - PubMed
- Tan PK; Liu S; Gunic E; Miner JN Sci. Rep 2017, 7, 665; - PMC - PubMed
- Liu L; Stelmach JE; Swaminathan R; Natarajan R; Chen MH; Singh SB; Schwartz CD; Fitzgerald CE; O’Keefe SJ; Zaller DM; Schmatz DM; Doherty JB Bioorg. Med. Chem. Lett 2003, 13, 3979; - PubMed
- Berndt A; Miller S; Williams O; Le DD; Houseman BT; Pacold JI; Gorrec F; Hon WC; Ren P; Liu Y; Rommel C; Gaillard P; Ruckle T; Schwarz MK; Shokat KM; Shaw JP; Williams RL Nat. Chem. Biol 2011, 6, 117; - PMC - PubMed
- Hopkins AL; Ren J; Milton J; Hazen RJ; Chan JH; Stuart DI; Stammers DK J. Med. Chem 2004, 47, 5912. - PubMed
-
- Beletskaya IP; Ananikov VP Chem. Rev 2011, 111, 1596; - PubMed
- Lee C-F; Liu Y-C, Badsara S Chem. Asian J 2014, 9, 706; - PubMed
- Ling R; Yoshida M; Mariano PS J. Org. Chem 1996, 61, 4439–4449; - PubMed
- Sujatha A; Thomas AM; Thankachan AP; Anilkumar G Arkivoc 2014, 2015, 1;
- Hartwig JF Acc. Chem. Rev 2008, 41, 1534; - PMC - PubMed
- Murata M; Buchwald SL Tetrahedron 2004, 60, 7397;
- Sayah M; Organ MG Chem. Eur. J 2011, 17, 11719; - PubMed
- Oderinde MS; Frenette M; Robbins DW; Aquila B; Johannes JW J. Am. Chem. Soc 2016, 138, 1760; - PubMed
- Jouffroy M; Kelly CB; Molander GA Org. Lett 2016, 18, 876. - PMC - PubMed
-
- Representative examples of SNAr reactions: Zhang X; Lu G-P; Cai C Green Chem 2016, 18, 5580;
- Beugelmans R; Bois-Choussy M; Boudet B Tetrahedron 1983, 39, 4153;
- Furukawa N; Ogawa S; Kawai T J. Chem. Soc. Perkin Trans. 1 1984, 1839;
- Bhagwat A; Campi EM; Potdar MK; Jackson RW; Hearn MTW Green Chem. Lett. Rev 2012, 5, 595;
- Omar WAE; Heiskanen JP; Hormi OEO J. Heterocyclic Chem 2008, 45, 593;
- Takashiro E; Nakamura Y; Fujimoto K Tetrahedron Lett 1999, 40, 5565;
- Cherng Y-J Tetrahedron 2002, 58, 4931.
-
- Hilton MC; Dolewski RD; McNally A J. Am. Chem. Soc 2016, 138, 13806; - PubMed
- Zhang X; McNally A Angew. Chem. Int. Ed 2017, 56, 9833; - PubMed
- Anders E; Markus F Tetrahedron Lett 1987, 28, 2675.
- Anders E; Markus F Chem. Ber 1989, 122, 112;
- Anders E; Markus F Chem. Ber 1989, 122, 119;
- Haas M; Goerls H; Anders E Synthesis 1998, 195;
- Bull JA; Mousseau JJ; Pelletier G; Charette AB Chem. Rev. 2012, 112, 2642. - PubMed
- Sammes MP; Leung CWF; Mak CK; Katritzky AR J. Chem. Soc. Perkin Trans. 1 1981, 1585.
-
- Finer J-P Ligand Coupling Reactions with Heteroaromatic Compounds. Tetrahedron Organic Chemistry Series; Pergamon Press: Oxford, 2009; Vol. 18, p 95;
- Byrne PA; Ortin Y; Gilheany DG Chem. Commun 2014, 51, 1147. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials