Action mechanism of bleaching herbicide cyclopyrimorate, a novel homogentisate solanesyltransferase inhibitor
- PMID: 30479543
- PMCID: PMC6240781
- DOI: 10.1584/jpestics.D18-008
Action mechanism of bleaching herbicide cyclopyrimorate, a novel homogentisate solanesyltransferase inhibitor
Abstract
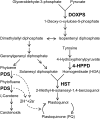
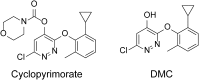
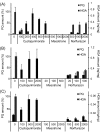
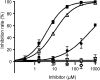
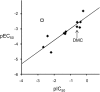
The action mechanism of cyclopyrimorate, a novel herbicide for weed control in rice fields, was investigated. Cyclopyrimorate caused bleaching symptoms in Arabidopsis thaliana similar to those caused by existing carotenoid biosynthesis inhibitors, mesotrione and norflurazon. However, cyclopyrimorate treatment resulted in significant accumulation of homogentisate and a reduction in the level of plastoquinone. A metabolite of cyclopyrimorate, des-morpholinocarbonyl cyclopyrimorate (DMC), was detected in plants. These data suggested that cyclopyrimorate and/or DMC inhibit homogentisate solanesyltransferase (HST), a downstream enzyme of 4-hydroxyphenylpyruvate dioxygenase in the plastoquinone biosynthesis pathway. In vitro assays showed that A. thaliana HST was strongly inhibited by DMC and weakly by cyclopyrimorate, whereas other commercial bleaching herbicides did not inhibit HST. DMC derivatives showed a positive correlation between HST inhibition and in vivo bleaching activities. These results indicate that the target site of cyclopyrimorate and DMC is HST, a novel target site of commercial herbicides.
Keywords: bleaching herbicide; carotenoid biosynthesis inhibitor; cyclopyrimorate; homogentisate solanesyltransferase; plastoquinone biosynthesis inhibitor.
Figures

References
-
- I. Heap: “Integrated Pest Management,” Springer, Netherlands, Chap. 12, pp. 281–301, 2014.
-
- http://www.weedscience.org/ (Accessed 21 Nov., 2017)
-
- S. O. Duke: Pest Manag. Sci. 68, 505–512 (2012). - PubMed
-
- http://hracglobal.com/ (Accessed 21 Nov., 2017)
-
- P. Boger and G. Sandmann: “Target Sites of Herbicide Action,” CRC Press, Boca Raton, FL, pp. 25–44, 1989.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
