Pharmacovigilance of medicines for rare and ultrarare diseases
- PMID: 30479738
- PMCID: PMC6243425
- DOI: 10.1177/2042098618792502
Pharmacovigilance of medicines for rare and ultrarare diseases
Abstract
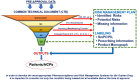
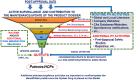
The assessment of the safety of medicines for rare diseases during the development phase is often limited by the few data available from small numbers of patients. This also applies to a lesser extent during the postmarketing phase of the lifecycle of a medicine. By using all available sources of data for rare diseases drugs, and by carefully assessing these data, the most informed safety profile can be obtained. This should also allow a clear view of data that are not available at any given time point and facilitates planning of strategies to obtain data through appropriate postmarketing risk management. Although it is not always easy, there are possibilities to increase the speed by which data in the postmarketing period can be generated by better use of data from ongoing formal clinical trials, by early planning of drug or disease registries and leveraging the power of both disease patient support groups, which are often well established, and networks to facilitate international research, specifically in rare diseases. The future may offer approaches using personal medical monitoring data tools and 'big data' to further facilitate the availability of information and to determine the effectiveness and safety profiles of drugs used for rare diseases and thus allow the benefit/risk of these drugs to be optimized. These issues will be discussed here.
Keywords: advanced therapy; drug safety; orphan drugs; pharmacovigilance; rare condition; rare diseases; rare disorder; risk management; signal detection; ultrarare disease.
Conflict of interest statement
Conflict of interest statement: The authors declare that there is no conflict of interest.
Figures
References
-
- European Medicines Agency. PRIME: priority medicines, http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/gen... (2016, accessed 29 May 2018).
-
- Luzzatto L, Hollak CE, Cox TM, et al. Rare diseases and effective treatments: are we delivering? Lancet 2015; 385: 750–752. - PubMed
-
- Kaspar RL. Challenges in developing therapies for rare diseases including pachyonychia congenita. J Investig Dermatol Symp Proc 2005; 10: 62–66. - PubMed
-
- Richter T, Nestler-Parr S, Babela R, et al. Rare disease terminology and definitions. A systematic global review: report of the ISPOR Rare Disease Special Interest Group. Value Health 2015; 18: 906–914. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

