Silicon-Enhanced Adipogenesis and Angiogenesis for Vascularized Adipose Tissue Engineering
- PMID: 30479923
- PMCID: PMC6247030
- DOI: 10.1002/advs.201800776
Silicon-Enhanced Adipogenesis and Angiogenesis for Vascularized Adipose Tissue Engineering
Abstract
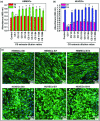
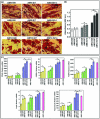
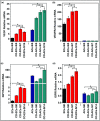
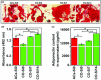
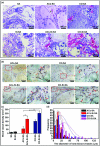
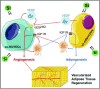
The enhancement of adipogenic differentiation of bone marrow mesenchymal stem cells (BMSCs) and sufficient vascularization remain great challenges for the successful reconstruction of engineered adipose tissue. Here, the bioactive effects of silicon (Si) ions on adipogenic differentiation of human BMSCs (HBMSCs) and the stimulation of vascularization during adipose tissue regeneration are reported. The results show that Si ions can enhance adipogenic differentiation of HBMSCs through the stimulation of the expression of adipogenic differentiation switches such as peroxisome proliferator-activated receptor γ and CCAAT/enhancer-binding protein α. Furthermore, Si ions can enhance both angiogenesis and adipogenesis, and inhibit dedifferentiation of cocultured adipocytes by regulating the interactions between HBMSC-derived adipocytes and human umbilical vein endothelial cells, in which the promotion of the expression of insulin-like growth factor 1 and vascular endothelial growth factor plays vital roles. The in vivo studies further demonstrate that the designed composite hydrogel with the ability to release bioactive Si ions clearly stimulates neovascularization and adipose tissue regeneration. The study suggests that Si ions released from biomaterials are important chemical cues for adipogenic differentiation and biomaterials with the ability to release Si ions can be designed for adipose tissue engineering.
Keywords: adipogenesis; adipogenic differentiation; adipose tissue engineering; angiogenesis; dedifferentiation.
Figures



Similar articles
-
Chemokine (C-C motif) ligand 2-enhanced adipogenesis and angiogenesis of human adipose-derived stem cell and human umbilical vein endothelial cell co-culture system in adipose tissue engineering.J Tissue Eng Regen Med. 2022 Feb;16(2):163-176. doi: 10.1002/term.3264. Epub 2021 Dec 1. J Tissue Eng Regen Med. 2022. PMID: 34811942
-
Bone tissue engineering strategy based on the synergistic effects of silicon and strontium ions.Acta Biomater. 2018 May;72:381-395. doi: 10.1016/j.actbio.2018.03.051. Epub 2018 Apr 6. Acta Biomater. 2018. PMID: 29627679
-
Knockdown of CDC20 promotes adipogenesis of bone marrow-derived stem cells by modulating β-catenin.Stem Cell Res Ther. 2022 Sep 2;13(1):443. doi: 10.1186/s13287-022-03062-0. Stem Cell Res Ther. 2022. PMID: 36056439 Free PMC article.
-
Adipose-derived stem cell differentiation as a basic tool for vascularized adipose tissue engineering.Differentiation. 2016 Jul-Aug;92(1-2):52-64. doi: 10.1016/j.diff.2016.02.003. Epub 2016 Mar 11. Differentiation. 2016. PMID: 26976717 Review.
-
Molecular Regulation of Adipogenesis and Potential Anti-Adipogenic Bioactive Molecules.Int J Mol Sci. 2016 Jan 19;17(1):124. doi: 10.3390/ijms17010124. Int J Mol Sci. 2016. PMID: 26797605 Free PMC article. Review.
Cited by
-
Nanostructured 3D-Printed Hybrid Scaffold Accelerates Bone Regeneration by Photointegrating Nanohydroxyapatite.Adv Sci (Weinh). 2023 May;10(13):e2300038. doi: 10.1002/advs.202300038. Epub 2023 Mar 11. Adv Sci (Weinh). 2023. PMID: 36905235 Free PMC article.
-
Nano-calcium silicate mineralized fish scale scaffolds for enhancing tendon-bone healing.Bioact Mater. 2022 May 18;20:29-40. doi: 10.1016/j.bioactmat.2022.04.030. eCollection 2023 Feb. Bioact Mater. 2022. PMID: 35633872 Free PMC article.
-
The combination of multi-functional ingredients-loaded hydrogels and three-dimensional printed porous titanium alloys for infective bone defect treatment.J Tissue Eng. 2020 Oct 23;11:2041731420965797. doi: 10.1177/2041731420965797. eCollection 2020 Jan-Dec. J Tissue Eng. 2020. PMID: 33149880 Free PMC article.
-
Tailoring and characterization of bioactive graft material for alveolar bone preservation and regeneration in fresh extraction sockets of dog model.Sci Rep. 2025 Jan 27;15(1):3321. doi: 10.1038/s41598-025-86408-x. Sci Rep. 2025. PMID: 39865138 Free PMC article.
-
Asymmetric adhesive SIS-based wound dressings for therapeutically targeting wound repair.J Nanobiotechnology. 2024 Jan 19;22(1):34. doi: 10.1186/s12951-024-02294-x. J Nanobiotechnology. 2024. PMID: 38238748 Free PMC article.
References
-
- a) Choi J. H., Gimble J. M., Lee K., Marra K. G., Rubin J. P., Yoo J. J., Vunjak‐Novakovic G., Kaplan D. L., Tissue Eng., Part B 2010, 16, 413; - PMC - PubMed
- b) Huber B., Volz A. C., Kluger P. J., Cell Tissue Res. 2015, 362, 269; - PubMed
- c) Volz A. C., Huber B., Kluger P. J., Differentiation 2016, 92, 52. - PubMed
-
- a) Ninomiya Y., Sugahara‐Yamashita Y., Nakachi Y., Tokuzawa Y., Okazaki Y., Nishiyama M., Biochem. Biophys. Res. Commun. 2010, 394, 303; - PubMed
- b) Gomillion C. T., Burg K. J., Biomaterials 2006, 27, 6052; - PubMed
- c) Masuda T., Furue M., Matsuda T., Tissue Eng. 2004, 10, 523; - PubMed
- d) Scott M. A., Nguyen V. T., Levi B., James A. W., Stem Cells Dev. 2011, 20, 1793. - PMC - PubMed
-
- Mauney J. R., Volloch V., Kaplan D. L., Biomaterials 2005, 26, 6167. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
