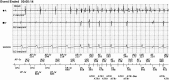
Respiratory rate trending as a cause for atrial lead noise: A first report in an implantable cardioverter-defibrillator patient
- PMID: 30479957
- PMCID: PMC6241333
- DOI: 10.1016/j.hrcr.2018.08.007
Respiratory rate trending as a cause for atrial lead noise: A first report in an implantable cardioverter-defibrillator patient
Keywords: Artifact; Device lead mismatch; High impedance; Minute ventilation sensor; Oversensing.
Figures
References
-
- Maisel W.H., Sweeney M.O., Stevenson W.G., Ellison K.E., Epstein L.M. Recalls and safety alerts involving pacemakers and implantable cardioverter-defibrillator generators. JAMA. 2001;286:793–799. - PubMed
-
- Gould P.A., Krahn A.D., Canadian Heart Rhythm Society Working Group on Device Advisories Complications associated with implantable cardioverter-defibrillator replacement in response to device advisories. JAMA. 2006;295:1907–1911. - PubMed
-
- Minute Ventilation Signal Oversensing Physician Letter - December 2017, 2018. http://www.bostonscientific.com/en-US/pprc/product-advisories.html
-
- FDA Class 2 Device Recall - Boston Scientific Pacemakers. 2018. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRES/res.cfm?start_s...
-
- Hansen C., Vollmann D., Neuzner J. Device malfunction caused by “auto-oversensing” of transthoracic impedance measurement test pulses in a modern minute ventilation dual-chamber pacemaker. Clin Res Cardiol. 2016;105:571–574. - PubMed
Publication types
LinkOut - more resources
Full Text Sources