Randomised controlled trial of cognitive behavioural therapy in COPD
- PMID: 30479999
- PMCID: PMC6250562
- DOI: 10.1183/23120541.00094-2018
Randomised controlled trial of cognitive behavioural therapy in COPD
Abstract
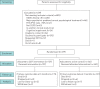
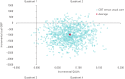
Anxiety is an important comorbidity in chronic obstructive pulmonary disease (COPD). We investigated if cognitive behavioural therapy (CBT), delivered by respiratory nurses, reduced symptoms of anxiety and was cost-effective. Patients with COPD and anxiety were randomised to CBT or self-help leaflets. Anxiety, depression and quality of life were measured at baseline, 3, 6 and 12 months. A cost-effectiveness analysis was conducted from a National Health Service hospital perspective and quality-adjusted life-years estimated using the EuroQol-5D questionnaire. In total, 279 patients were recruited. Group mean change from baseline to 3 months in the Hospital Anxiety and Depression Anxiety Subscale was 3.4 (95% CI 2.62-4.17, p<0.001) for the CBT group and 1.88 (95% CI 1.19-2.55, p<0.001) in the leaflet group. The CBT group was superior to leaflets at 3 months (mean difference in the Hospital Anxiety and Depression Anxiety Subscale was 1.52, 95% CI 0.49-2.54, p=0.003). Importantly, the CBT intervention was more cost-effective than leaflets at 12 months, significantly lowering hospital admissions and attendance at emergency departments. CBT delivered by respiratory nurses is a clinically and cost-effective treatment for anxiety in patients with COPD relative to self-help leaflets.
Conflict of interest statement
Conflict of interest: D. Carrick-Sen has nothing to disclose. Conflict of interest: C. Baker reports grants from National Institute for Health Research (NIHR)/Health Education England (support for part-time PhD fellowship for K. Heslop-Marshall), non-financial support from University of Newcastle upon Tyne (advice for Nick Steen, Medical Statistician, Newcastle University), during the conduct of the study. Conflict of interest: J. Gray has nothing to disclose. Conflict of interest: J. Newton has nothing to disclose. Conflict of interest: C. Stenton has nothing to disclose. Conflict of interest: M. Jambon has nothing to disclose. Conflict of interest: K. Pearce has nothing to disclose. Conflict of interest: K. Heslop-Marshall reports grants from National Institute for Health Research (UK), during the conduct of the study; and other from Pivotal Health Education Ltd, outside the submitted work. (This research was completed in February 2016. In 2017, K. Heslop-Marshall set up a company to provide cognitive behavioural therapy (CBT) training for healthcare professionals who would like to learn foundation level skills in CBT to work with patients with long-term conditions who have psychological distress. This training will not just benefit professionals working in the respiratory setting. I did this to enhance the psychological skills and pass on my knowledge and expertise in CBT. This did not influence the research submitted in any way.) Conflict of interest: G. Burns reports personal fees from BI, Chiesi and AZ (for advice on inhalers, not directly relevant to this study), personal fees from Teva (for three educational (non-promotional) talks on COPD, not related to this study), personal fees from Chiesi (for three educational (non-promotional) talks on COPD, not related to this study), personal fees from Pfizer and AZ (for educational (non-promotional) talks on COPD, not related to this study), all outside the submitted work. Conflict of interest: A. De Soyza reports other from AstraZeneca (bronchiectasis interest group meeting support), non-financial support from Novartis (in kind support for analysis in bronchiectasis), non-financial support from Forest labs (bronchiectasis interest group meeting support), personal fees from Bayer (speakers’/advisory boards on bronchiectasis), personal fees and other from Novartis (advisory boards on bronchiectasis and in kind bench science support), other from Chiesi (travel bursary to attend ERS meeting), other from Almirall (travel bursary to attend ERS meeting), other from Boehringer Ingelheim (travel bursary to attend BTS meeting), personal fees from AstraZeneca (speakers’/advisory boards on COPD), grants from AstraZeneca (travel bursary to attend ATS meeting), all outside the submitted work. In addition, A. De Soyza has received medical education grant support for a UK bronchiectasis network from GSK, Gilead, Chiesi and Forest labs. A. De Soyza's employing institution receives fees for his work as Coordinating investigator in a phase III trial in Bronchiectasis sponsored by Bayer. Conflict of interest: C. Echevarria has nothing to disclose.
Figures
Comment in
-
Nurse-led cognitive behavioural therapy for treatment of anxiety in COPD.ERJ Open Res. 2018 Dec 14;4(4):00221-2018. doi: 10.1183/23120541.00221-2018. eCollection 2018 Oct. ERJ Open Res. 2018. PMID: 30568966 Free PMC article.
References
-
- Global Initiative for Chronic Obstructive Lung Disease Global Strategy for the Prevention, Diagnosis and Management of COPD. 2017. www.goldcopd.org
-
- Yohannes AM, Kaplan A, Hanania NA. Anxiety and depression in chronic obstructive pulmonary disease: recognition and management. Cleve Clin J Med 2018: S11–SS8. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
