A Difficult Challenge for the Clinical Laboratory: Accessing and Interpreting Manufacturer Cross-Reactivity Data for Immunoassays Used in Urine Drug Testing
- PMID: 30480089
- PMCID: PMC6249658
- DOI: 10.1177/2374289518811797
A Difficult Challenge for the Clinical Laboratory: Accessing and Interpreting Manufacturer Cross-Reactivity Data for Immunoassays Used in Urine Drug Testing
Abstract
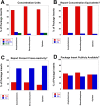
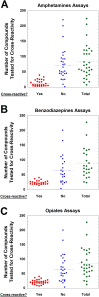
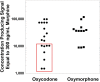
Urine drug testing by immunoassay is widely used to detect nonmedical drug use and to monitor patients prescribed controlled substances. A key attribute of urine drug testing immunoassays is cross-reactivity, namely the response of various compounds compared to the target of the assay. In this report, we analyzed the variability in how manufacturer cross-reactivity data are summarized in package inserts for commercially available amphetamines, benzodiazepines, and opiates immunoassays, 3 broad drug classes commonly included in routine drug testing panels. Specifically, we determined the number of compounds tested for cross-reactivity, manner in which cross-reactivity is measured, concentration units used, how often compounds known to be cross-reactive with marketed urine drug testing immunoassays prior to 2010 were tested, availability of the package insert online, and how often cross-reactivity on "designer drugs" was found in the package inserts. There was wide variability in the number of compounds tested (both positive and negative), with the highest number of tested compounds generally found in point-of-care urine drug testing applications. Most package inserts used ng/mL as the concentration units and expressed cross-reactivity in terms of equivalent concentrations to the assay calibrator. Approximately 50% of package inserts were directly available online. Cross-reactivity data were sparse with respect to "off-target" drugs known to be cross-reactive prior to 2010 (an example being quinolone antibiotics and opiates immunoassays) and designer drugs. The present study indicates lack of consistency in cross-reactivity information in package inserts, complicating the interpretation of urine drug testing results. We use 3 example clinical cases to illustrate practical challenges accessing and interpreting cross-reactivity data.
Keywords: amphetamines; benzodiazepines; designer drugs; false positives; immunoassay; opiates.
Conflict of interest statement
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
References
-
- Akerele E, Olupona T. Drugs of abuse. Psychiatr Clin North Am. 2017;40:501–517. - PubMed
-
- Lipari RN, Van Horn SL. Trends in Substance Use Disorders Among Adults Aged 18 or Older. CBHSQ Report. Rockville, MD: Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality; 2013:1–10. - PubMed
-
- Pergolizzi JV, Jr, LeQuang JA, Taylor R, Jr, Raffa RB; NEMA Research Group. Going beyond prescription pain relievers to understand the opioid epidemic: the role of illicit fentanyl, new psychoactive substances, and street heroin. Postgrad Med. 2018;130:1–8. - PubMed
LinkOut - more resources
Full Text Sources

