Omega-3 fatty acid addition during pregnancy
- PMID: 30480773
- PMCID: PMC6516961
- DOI: 10.1002/14651858.CD003402.pub3
Omega-3 fatty acid addition during pregnancy
Abstract
Background: Higher intakes of foods containing omega-3 long-chain polyunsaturated fatty acids (LCPUFA), such as fish, during pregnancy have been associated with longer gestations and improved perinatal outcomes. This is an update of a review that was first published in 2006.
Objectives: To assess the effects of omega-3 LCPUFA, as supplements or as dietary additions, during pregnancy on maternal, perinatal, and neonatal outcomes and longer-term outcomes for mother and child.
Search methods: For this update, we searched Cochrane Pregnancy and Childbirth's Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (16 August 2018), and reference lists of retrieved studies.
Selection criteria: Randomised controlled trials (RCTs) comparing omega-3 fatty acids (as supplements or as foods, stand-alone interventions, or with a co-intervention) during pregnancy with placebo or no omega-3, and studies or study arms directly comparing omega-3 LCPUFA doses or types. Trials published in abstract form were eligible for inclusion.
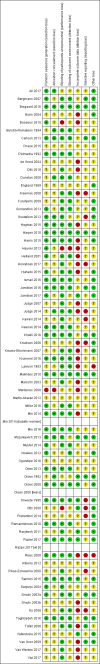
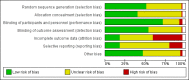
Data collection and analysis: Two review authors independently assessed study eligibility, extracted data, assessed risk of bias in trials and assessed quality of evidence for prespecified birth/infant, maternal, child/adult and health service outcomes using the GRADE approach.
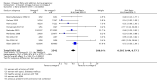
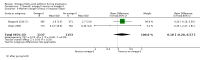
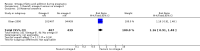
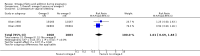
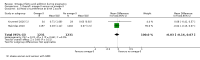
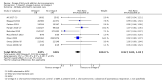
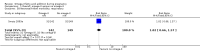
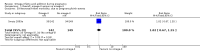
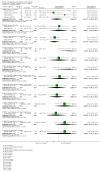
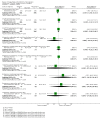
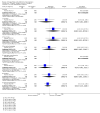
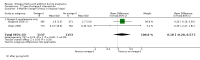
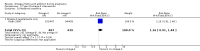
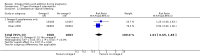
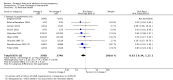
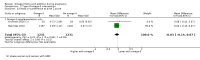
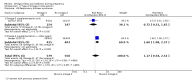
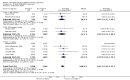
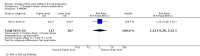
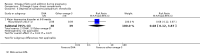
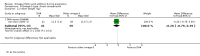
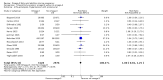
Main results: In this update, we included 70 RCTs (involving 19,927 women at low, mixed or high risk of poor pregnancy outcomes) which compared omega-3 LCPUFA interventions (supplements and food) compared with placebo or no omega-3. Overall study-level risk of bias was mixed, with selection and performance bias mostly at low risk, but there was high risk of attrition bias in some trials. Most trials were conducted in upper-middle or high-income countries; and nearly half the trials included women at increased/high risk for factors which might increase the risk of adverse maternal and birth outcomes.Preterm birth < 37 weeks (13.4% versus 11.9%; risk ratio (RR) 0.89, 95% confidence interval (CI) 0.81 to 0.97; 26 RCTs, 10,304 participants; high-quality evidence) and early preterm birth < 34 weeks (4.6% versus 2.7%; RR 0.58, 95% CI 0.44 to 0.77; 9 RCTs, 5204 participants; high-quality evidence) were both lower in women who received omega-3 LCPUFA compared with no omega-3. Prolonged gestation > 42 weeks was probably increased from 1.6% to 2.6% in women who received omega-3 LCPUFA compared with no omega-3 (RR 1.61 95% CI 1.11 to 2.33; 5141 participants; 6 RCTs; moderate-quality evidence).For infants, there was a possibly reduced risk of perinatal death (RR 0.75, 95% CI 0.54 to 1.03; 10 RCTs, 7416 participants; moderate-quality evidence: 62/3715 versus 83/3701 infants) and possibly fewer neonatal care admissions (RR 0.92, 95% CI 0.83 to 1.03; 9 RCTs, 6920 participants; moderate-quality evidence - 483/3475 infants versus 519/3445 infants). There was a reduced risk of low birthweight (LBW) babies (15.6% versus 14%; RR 0.90, 95% CI 0.82 to 0.99; 15 trials, 8449 participants; high-quality evidence); but a possible small increase in large-for-gestational age (LGA) babies (RR 1.15, 95% CI 0.97 to 1.36; 6 RCTs, 3722 participants; moderate-quality evidence, for omega-3 LCPUFA compared with no omega-3. Little or no difference in small-for-gestational age or intrauterine growth restriction (RR 1.01, 95% CI 0.90 to 1.13; 8 RCTs, 6907 participants; moderate-quality evidence) was seen.For the maternal outcomes, there is insufficient evidence to determine the effects of omega-3 on induction post-term (average RR 0.82, 95% CI 0.22 to 2.98; 3 trials, 2900 participants; low-quality evidence), maternal serious adverse events (RR 1.04, 95% CI 0.40 to 2.72; 2 trials, 2690 participants; low-quality evidence), maternal admission to intensive care (RR 0.56, 95% CI 0.12 to 2.63; 2 trials, 2458 participants; low-quality evidence), or postnatal depression (average RR 0.99, 95% CI 0.56 to 1.77; 2 trials, 2431 participants; low-quality evidence). Mean gestational length was greater in women who received omega-3 LCPUFA (mean difference (MD) 1.67 days, 95% CI 0.95 to 2.39; 41 trials, 12,517 participants; moderate-quality evidence), and pre-eclampsia may possibly be reduced with omega-3 LCPUFA (RR 0.84, 95% CI 0.69 to 1.01; 20 trials, 8306 participants; low-quality evidence).For the child/adult outcomes, very few differences between antenatal omega-3 LCPUFA supplementation and no omega-3 were observed in cognition, IQ, vision, other neurodevelopment and growth outcomes, language and behaviour (mostly low-quality to very low-quality evidence). The effect of omega-3 LCPUFA on body mass index at 19 years (MD 0, 95% CI -0.83 to 0.83; 1 trial, 243 participants; very low-quality evidence) was uncertain. No data were reported for development of diabetes in the children of study participants.
Authors' conclusions: In the overall analysis, preterm birth < 37 weeks and early preterm birth < 34 weeks were reduced in women receiving omega-3 LCPUFA compared with no omega-3. There was a possibly reduced risk of perinatal death and of neonatal care admission, a reduced risk of LBW babies; and possibly a small increased risk of LGA babies with omega-3 LCPUFA.For our GRADE quality assessments, we assessed most of the important perinatal outcomes as high-quality (e.g. preterm birth) or moderate-quality evidence (e.g. perinatal death). For the other outcome domains (maternal, child/adult and health service outcomes) GRADE ratings ranged from moderate to very low, with over half rated as low. Reasons for downgrading across the domain were mostly due to design limitations and imprecision.Omega-3 LCPUFA supplementation during pregnancy is an effective strategy for reducing the incidence of preterm birth, although it probably increases the incidence of post-term pregnancies. More studies comparing omega-3 LCPUFA and placebo (to establish causality in relation to preterm birth) are not needed at this stage. A further 23 ongoing trials are still to report on over 5000 women, so no more RCTs are needed that compare omega-3 LCPUFA against placebo or no intervention. However, further follow-up of completed trials is needed to assess longer-term outcomes for mother and child, to improve understanding of metabolic, growth and neurodevelopment pathways in particular, and to establish if, and how, outcomes vary by different types of omega-3 LCPUFA, timing and doses; or by characteristics of women.
Conflict of interest statement
Philippa Middleton: none known.
Judith C Gomersall: none known.
Emily Shepherd: none known.
Sjurdur F Olsen: Sjurdur Olsen’s institution has received grant funding from the Innovation Fund Denmark (grant no. 09‐067124, Centre for Fetal Programming) and from the March of Dimes Foundation (6‐FY‐96‐0240, 6‐FY97‐0553, 6‐FY97‐0521, 6‐FY00‐407). Sjurdur Olsen was the principal investigator of three trials included in the review: Olsen 1992, Olsen 2000 and Knudsen 2006.
Maria Makrides: Maria Makrides’ institution has received grant funding (NHMRC Fellowship 1061704 Improving the outcomes of mothers and babies through nutritional interventions and NHMRC 1146806 Efficacy and safety of omega‐3 DHA supplementation in preterm infants: childhood follow‐up of the N3RO trial); and honorarium money from Fonterra (for advice relating to maternal and child nutrition) to fund ECR and MCR conference travel. Professor Makrides is the principal investigator of the DOMInO trial (included) and the ORIP (ongoing) trial trials. She was on the Scientific Advisory Board of Fonterra (to September 2018); and she is President Elect of the International Society for the Study of Fatty Acids and Lipids (ISSFAL).
Jacqueline Gould is an investigator on some of the DOMInO follow‐up studies. She has received honoraria from the Nestle Nutrition Institute ‐ these have been paid to Dr Gould’s institution to support conference travel.
Dr Gould is an investigator in a clinical trial of high‐dose DHA supplementation during pregnancy that was eligible for inclusion in this review. Dr Gould's institute has received project grant funding and a fellowship from the NHMRC and WCH Foundation for work unrelated to this review. She has received honoraria from the Nestle Nutrition Institute and Fonterra ‐ these have been paid to Dr Gould’s institution to support conference travel.
Sjurdur Olsen, Maria Makrides and Jacqueline Gould were not involved in any decisions relating to the above studies and assessment for inclusion/exclusions, risk of bias, data extraction, etc. was carried out by other members of the review team who were not directly involved in these studies.
Figures


























































































































































































































Update of
-
Marine oil, and other prostaglandin precursor, supplementation for pregnancy uncomplicated by pre-eclampsia or intrauterine growth restriction.Cochrane Database Syst Rev. 2006 Jul 19;(3):CD003402. doi: 10.1002/14651858.CD003402.pub2. Cochrane Database Syst Rev. 2006. Update in: Cochrane Database Syst Rev. 2018 Nov 15;11:CD003402. doi: 10.1002/14651858.CD003402.pub3. PMID: 16856006 Updated.
Comment in
-
Omega-3 Fatty Acid Addition During Pregnancy: Summary of a Cochrane Review.Explore (NY). 2019 Mar-Apr;15(2):168-169. doi: 10.1016/j.explore.2018.12.005. Epub 2019 Jan 3. Explore (NY). 2019. PMID: 30679097 No abstract available.
-
Increased intake of omega-3 during pregnancy can reduce preterm births.Evid Based Nurs. 2020 Apr;23(2):63. doi: 10.1136/ebnurs-2019-103064. Epub 2019 Jul 10. Evid Based Nurs. 2020. PMID: 31292152 No abstract available.
References
References to studies included in this review
Ali 2017 {published data only}
-
- Ali MK. The effect of omega 3 on pregnancy complicated by asymmetrical intrauterine growth restriction. clinicaltrials.gov/ct2/show/NCT02696577 (first received 28 February 2016).
-
- Ali MK, Amin ME, Amin AF, Abd El, Aal DE. Evaluation of the effectiveness of low‐dose aspirin and omega 3 in treatment of asymmetrically intrauterine growth restriction: a randomized clinical trial. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2017;210:231‐5. [NCT02696577] - PubMed
Bergmann 2007 {published data only}
-
- Bergmann RL, Bergmann KE, Haschke‐Becher E, Richter R, Dudenhausen JW, Barclay D, et al. Does maternal docosahexaenoic acid supplementation during pregnancy and lactation lower BMI in late infancy?. Journal of Perinatal Medicine 2007;35(4):295‐300. - PubMed
-
- Bergmann RL, Bergmann KE, Richter R, Haschke‐Becher E, Henrich W, Dudenhausen JW. Does docosahexaenoic acid (DHA) status in pregnancy have any impact on postnatal growth? Six‐year follow‐up of a prospective randomized double‐blind monocenter study on low‐dose DHA supplements. Journal of Perinatal Medicine 2012;40:677‐84. - PubMed
-
- Bergmann RL, Haschke‐Becher E, Klassen‐Wigger P, Bergmann KE, Richter R, Dudenhausen JW, et al. Supplementation with 200 mg/day docosahexaenoic acid from mid‐pregnancy through lactation improves the docosahexaenoic acid status of mothers with a habitually low fish intake and of their infants. Annals of Nutrition & Metabolism 2008;52(2):157‐66. - PMC - PubMed
-
- Bergmann RL, Haschke‐Becher, Bergmann KE, Dudenhausen JW, Haschke F. Low dose docosahexaenoic acid supplementation improves the DHA status of pregnant women. Pediatric Academic Societies Annual Meeting; 2006 April 29‐May 2; San Francisco (CA). 2006.
-
- Bergmann RL, Richter R, Bergmann KE, Dudenhausen JW, Haschke F. 21 months old infants are leaner if their mothers received low dose DHA supplements during pregnancy and lactation. European Journal of Pediatrics 2006;165(Suppl 1):114.
Bisgaard 2016 {published data only}
-
- Bisgaard H. Fish oil supplementation during pregnancy for prevention of asthma, eczema and allergies in childhood: interventional trial in the cCOPSAC2010 (Copenhagen studies on asthma in childhood) birth cohort. clinicaltrials.gov/ct2/show/NCT00798226 (accessed 15 September 2012).
-
- Bisgaard H, Stockholm J, Chawes B, Vissing N, Bjarndottir E, Schoos A, et al. Fish oil‐derived fatty acids in pregnancy and wheeze and asthma in offspring. New England Journal of Medicine 2016;375(26):2530‐8. - PubMed
Boris 2004 {published data only}
-
- Boris J, Jensen B, Salvig JD, Secher NJ, Olsen SF. A randomized controlled trial of the effect of fish oil supplementation in late pregnancy and early lactation on the n‐3 fatty acid content in human breast milk. Lipids 2004;39(12):1191‐6. - PubMed
Bosaeus 2015 {published data only}
Bulstra‐Ramakers 1994 {published data only}
-
- Bulstra‐Ramakers MT, Huisjes HJ, Visser GH. The effects of 3g eicosapentaenoic acid daily on recurrence of intrauterine growth retardation and pregnancy induced hypertension. British Journal of Obstetrics and Gynaecology 1994;102:123‐6. - PubMed
Carlson 2013 {published data only}
-
- Carlson S, NCT02487771. Kansas University DHA Outcome Study (KUDOS) follow‐up. clinicaltrials.gov/show/NCT02487771 (first received 1 July 2015).
-
- Carlson SE. DHA supplementation and pregnancy outcome. clinicaltrials.gov/ct2/show/NCT00266825 (first received 19 December 2005).
-
- Carlson SE, Gajewski BJ, Alhayek S, Colombo J, Kerling EH, Gustafson KM. Dose‐response relationship between docosahexaenoic acid (DHA) intake and lower rates of early preterm birth, low birth weight and very low birth weight. Prostaglandins, Leukotrienes and Essential Fatty Acids 2018;138:1‐5. - PMC - PubMed
Chase 2015 {published data only}
-
- Chase HP, Lescheck E, Rafkin‐Mervis L, Krause‐Steinrauf H, Chritton S, Azare SM, et al. Nutritional intervention to prevent (NIP) type 1 diabetes: a pilot trial. Childhood Obesity and Nutrition 2009;1(2):98‐107. [DOI: 10.1177/1941406409333466] - DOI
D'Almedia 1992 {published data only}
-
- D'Almeida A, Carter JP, Anatol A, Prost C. Effects of a combination of evening primrose oil (gamma linolenic acid) and fish oil (eicosapentaenoic + docahexaenoic acid) versus magnesium, and versus placebo in preventing pre‐eclampsia. Women & Health 1992;19:117‐31. - PubMed
de Groot 2004 {published data only}
-
- Otto SJ, Groot RH, Hornstra G. Increased risk of postpartum depressive symptoms is associated with slower normalization after pregnancy of the functional docosahexaenoic acid status. Prostaglandins, Leukotrienes, and Essential Fatty Acids 2003;69(4):237‐43. - PubMed
-
- Groot RH, Hornstra G, Jolles J. Exploratory study into the relation between plasma phospholipid fatty acid status and cognitive performance. Prostaglandins Leukotrienes and Essential Fatty Acids 2004;76(3):165‐72. - PubMed
-
- Groot RH, Hornstra G, Houwelingen AC, Roumen F. Effect of alpha‐linolenic acid supplementation during pregnancy on maternal and neonatal polyunsaturated fatty acid status and pregnancy outcome. American Journal of Clinical Nutrition 2004;79:251‐60. - PubMed
Dilli 2018 {published data only}
-
- Dilli D. Fish oil supplementation in women with gestational diabetes. clinicaltrials.gov/ct2/show/NCT02371343 (first received 19 February 2015). [NCT02371343]
-
- Dilli D, Dogan NN, Ipek MS, Cavus Y, Ceylaner S, Dogan H, et al. MaFOS‐GDM trial: maternal fish oil supplementation in women with gestational diabetes and cord blood DNA methylation at insulin like growth factor‐1 (IGF‐1) gene. Clinical Nutrition ESPEN 2018;23:73‐8. - PubMed
Dunstan 2008 {published data only}
-
- Barden AE, Dunstan JA, Beilin LJ, Prescott SL, Mori TA. n ‐ 3 fatty acid supplementation during pregnancy in women with allergic disease: effects on blood pressure, and maternal and fetal lipids. Clinical Science 2006;111(4):289‐94. - PubMed
-
- Barden AE, Mori TA, Dunstan JA, Taylor AL, Thornton CA, Croft KD, et al. Fish oil supplementation in pregnancy lowers f2‐isoprostanes in neonates at high risk of atopy. Free Radical Research 2004;38:233‐9. - PubMed
-
- Denburg JA, Hatfield HM, Cyr MM, Hayes L, Holt PG, Semhi R, et al. Fish oil supplementation in pregnancy modifies neonatal progenitors at birth in infants at risk of atopy. Pediatric Research 2005;57(2):276‐81. - PubMed
-
- Dunstan JA, Mitoulas LR, Dixon G, Doherty DA, Hartmann PE, Simmer K, et al. The effects of fish oil supplementation in pregnancy on breast milk fatty acid composition over the course of lactation: a randomized controlled trial. Pediatric Research 2007;62(6):689‐94. - PubMed
England 1989 {published data only}
-
- England MJ, Chetty N, Dukes IA, Reavis S, Atkinson P, Sonnendecker EW. Eicosapentaenoic acid in pre‐eclampsia: a randomized trial. Proceedings of Silver Jubilee Congress of Obstetrics and Gynaecology; 1989; London (UK). 1989:154.
Freeman 2008 {published data only}
-
- Freeman MP, Davis MF. Supportive psychotherapy for perinatal depression: preliminary data for adherence and response. Depression and Anxiety 2010;27(1):39‐45. - PubMed
-
- Freeman MP, Sinha P. Tolerability of omega‐3 fatty acid supplements in perinatal women. Prostaglandins, Leukotrienes and Essential Fatty Acids 2007;77(3‐4):203‐8. - PubMed
Furuhjelm 2009 {published data only}
-
- Duchen K. Omega‐3 fatty acid supplementation in pregnancy and during lactation: a randomized, double‐blind, placebo controlled trial. clinicaltrials.gov/show/NCT00892684 (first received 4 May 2009).
-
- Duchen KM. Combined dietary supplementation with Lactobacillus reuteri and omega‐3 PUFA during pregnancy and postnatally in relation to development of IgE‐associated disease during infancy. clinicaltrials.gov/show/NCT01542970 (first received 2 March 2012).
-
- Furuhjelm C, Jenmalm MC, Falth‐Magnusson K, DuchIn K. Th1 and Th2 chemokines, vaccine‐induced immunity, and allergic disease in infants after maternal ‐3 fatty acid supplementation during pregnancy and lactation. Pediatric Research 2011;69(3):259‐64. - PubMed
-
- Furuhjelm C, Warstedt K, Fageras M, Falth‐Magnusson K, Larsson J, Fredriksson M, et al. Allergic disease in infants up to 2 years of age in relation to plasma omega‐3 fatty acids and maternal fish oil supplementation in pregnancy and lactation. Pediatric Allergy and Immunology 2011;22(5):505‐14. - PubMed
-
- Furuhjelm C, Warstedt K, Fageras M, Falth‐Magnusson K, Larsson J, Fredriksson M, et al. A randomised placebo controlled study of omega‐3‐fatty acid supplementation during pregnancy and lactation. Skin sensitisation in the children. Allergy 2007;62(Suppl 83):46.
Giorlandino 2013 {published data only}
-
- Giorlandino C, ISRCTN39268609. Effect of vaginally administered docosahexaenoic acid (DHA) fatty acids on pregnancy outcome: a randomised controlled trial. isrctn.com/ISRCTN39268609 (first received 27 May 2009). [ISRCTN39268609]
Gustafson 2013 {published data only}
-
- Carlson SE, Gajewski BJ, Alhayek S, Colombo J, Kerling EH, Gustafson KM. Dose‐response relationship between docosahexaenoic acid (DHA) intake and lower rates of early preterm birth, low birth weight and very low birth weight. Prostaglandins, Leukotrienes and Essential Fatty Acids 2018;138:1‐5. - PMC - PubMed
-
- Gustafson K, NCT01007110. The effects of docosahexaenoic acid (DHA) on fetal cardiac outcomes. clinicaltrials.gov/show/NCT01007110 (first received 3 November 2009).
Haghiac 2015 {published data only}
-
- Calabuig‐Navarro V, Haghiac M, Catalano P, Hauguel de‐Mouzon S, O'Tierney‐Ginn P. Chronic omega‐3 supplementation during pregnancy is associated with decreases in lipid accumulation pathways in placenta of obese and overweight women. Reproductive Sciences 2015;22:289A.
-
- Calabuig‐Navarro V, Puchowicz M, Haghiac M, Catalano P, De‐Mouzon SH, O'Tierney‐Ginn P. Reduced fatty acid esterification and accumulation in placentas of omega‐3 supplemented women. Diabetes 2015;64:A95.
-
- Catalano P, Haghiac M, Smith S, Dettlebach S, Gunzler D, Groh‐Wargo S, et al. Omega‐3 poly‐unsaturated fatty acid supplementation in overweight and obese women: a pilot RCT to improve inflammation, insulin sensitivity and decrease fetal adiposity. American Journal of Obstetrics and Gynecology 2014;210(1 Suppl):S43.
Harper 2010 {published data only}
-
- Bustos ML, Caritis SN, Jablonski KA, Reddy UM, Sorokin Y, Manuck T, et al. The association among cytochrome P450 3A, progesterone receptor polymorphisms, plasma 17‐alpha hydroxyprogesterone caproate concentrations, and spontaneous preterm birth. American Journal of Obstetrics and Gynecology 2017;271(3):369.e1‐369.e9. [DOI: 10.1016/j.ajog.2017.05.019] - DOI - PMC - PubMed
-
- Caritis S. Pharmacodynamic impact of 17‐hydroxyprogesterone caproate (17‐OHPC) in singleton gestation. Reproductive Sciences 2012;19(3 Suppl):126A.
-
- Harper M. Low maternal omega‐3 levels prior to 22 weeks' gestation are associated with preterm delivery and low fish intake. American Journal of Obstetrics and Gynecology 2009;201(6 Suppl 1):S172‐3.
-
- Harper M. Omega‐3 fatty acids and cytokine production. American Journal of Obstetrics and Gynecology 2011;204(1 Suppl):S202.
Harris 2015 {published data only}
-
- Harris MA. DHA supplementation and pregnancy outcome. clinicaltrials.gov/ct2/show/record/NCT02219399 (first received 18 August 2014).
-
- Harris MA, Reece MS, McGregor JA, Wilson JW, Burke SM, Wheeler M, et al. The effect of omega‐3 docosahexaenoic acid supplementation on gestational length: randomized trial of supplementation compared to nutrition education for Increasing n‐3 intake from foods. BioMed Research International 2015;2015:Article ID: 123078. [CENTRAL: 3195090; DOI: 10.1155/2015/123078] - DOI - PMC - PubMed
Hauner 2012 {published data only}
-
- Amann‐Gassner U, NCT00362089. The impact of the nutritional fatty acids during pregnancy and lactation for early human adipose tissue development. clinicaltrials.gov/ct2/show/NCT00362089 (first received 3 February 2014).
-
- Brei C, Brunner S, Pusch K, Much D, Stecher L, Amann‐Gassner U, et al. Long‐chain polyunsaturated fatty acids during pregnancy/lactation and children's body composition: 5‐year follow‐up data (INFAT‐study). Annals of Nutrition and Metabolism 2015;67(Suppl 1):413.
-
- Brei C, Stecher L, Brunner S, Ensenauer R, Heinen F, Wagner PD, et al. Impact of the n‐6:n‐3 long‐chain PUFA ratio during pregnancy and lactation on offspring neurodevelopment: 5‐year follow‐up of a randomized controlled trial. European Journal of Clinical Nutrition 2017; Vol. 71, issue 9:1114‐20. [DOI: 10.1038/ejcn.2017.79] - DOI - PubMed
-
- Brei C, Stecher L, Much D, Karla MT, Amann‐Gass U, Shen J, et al. Reduction of the n‐6:n‐3 long‐chain PUFA ratio during pregnancy and childbirth on offspring body composition: follow‐up results from a randomized controlled trial up to 5 y of age. American Journal of Clinical Nutrition 2016; Vol. 103, issue 6:1472‐81. [DOI: ] - PubMed
Helland 2001 {published data only}
-
- Haugen G, Helland I. Influence of preeclampsia or maternal intake of omega‐3 fatty acids on the vasoactive effect of prostaglandin F‐two‐alpha in human umbilical arteries. Gynecologic & Obstetric Investigation 2001;52:75‐81. - PubMed
-
- Helland IB, Saugstad OD, Saarem K, Houwelingen AC, Nylander G, Drevon CA. Supplementation of n‐3 fatty acids during pregnancy and lactation reduces maternal plasma lipid levels and provides DHA to the infants. Journal of Maternal‐Fetal & Neonatal Medicine 2006;19(7):397‐406. - PubMed
-
- Helland IB, Saugstad OD, Smith L, Saarem K, Solvoll K, Ganes T, et al. Similar effects on infants of n‐3 and n‐6 fatty acid supplementation to pregnant and lactating women. Pediatrics 2001;108:1‐7. - PubMed
-
- Helland IB, Smith L, Blomen B, Saarem K, Saugstad OD, Drevon CA. Effect of supplementing pregnant and lactating mothers with n‐3 very‐long‐chain fatty acids on children's IQ and body mass index at 7 years of age. Pediatrics 2008;122(2):e472‐9. - PubMed
-
- Helland IB, Smith L, Saarem K, Saugstad OD, Drevon CA. Maternal supplementation with very‐long‐chain n‐3 fatty acids during pregnancy and lactation augments children's IQ at 4 years of age. Pediatrics 2003;111:e39‐44. - PubMed
Horvaticek 2017 {published data only}
-
- Djelmis J, ISRCTN15203878. The impact of EPA and DHA supplementation on C‐peptide preservation in type 1 diabetic pregnant women. isrctn.com/ISRCTN15203878 (first received 8 September 2016). [ISRCTN15203878]
-
- Horvaticek M, Djelmis J, Ivanisevic M, Oreskovic S, Herman M. Effect of eicosapentaenoic acid and docosahexaenoic acid supplementation on C‐peptide preservation in pregnant women with type‐1 diabetes: randomized placebo controlled clinical trial. European Journal of Clinical Nutrition 2017; Vol. 71, issue 8:968‐72. - PubMed
Hurtado 2015 {published data only}
-
- Campos‐Martinez A, Serrano L, Medina M, Ochoa J, Pena‐Caballero M. Levels of docosahexaenoic acid in pregnant women and their children after taking a fish oil enriched diet. Journal of Maternal‐Fetal and Neonatal Medicine 2012;25(S2):92.
-
- Diaz‐Castro J, Moreno‐Fernandez J, Hijano S, Kajarabille N, Pulido‐Moran M, Latunde‐Dada GO, et al. DHA supplementation: a nutritional strategy to improve prenatal Fe homeostasis and prevent birth outcomes related with Fe‐deficiency. Journal of Functional Foods 2015;19:385‐93.
-
- Hurtado JA, Iznaola C, Pena M, Ruiz J, Pena‐Quintana L, Kajarabille N, et al. Effects of maternal omega‐3 supplementation on fatty acids and on visual and cognitive development. Journal of Pediatric Gastroenterology and Nutrition 2015;61:472‐80. - PubMed
-
- Kajarabille N, Rodriguez Y, Hurtado J, Pena M, Pena‐Quintana L, Lage S, et al. Effect of DHA supplementation on inflammatory signaling in pregnant women and their neonates. Annals of Nutrition and Metabolism 2013;63(Suppl 1):580.
Ismail 2016 {published data only}
-
- Ismail AA, NCT01990690. Role of antioxidants in unexplained oligohydramnios, a randomized trial. clinicaltrials.gov/ct2/show/NCT01990690 (first received 21 November 2013). [NCT01990690]
-
- Ismail AA, Ramadan MF, Ali MK, Abbas AM, Saman AM, Makarem MH. A randomized controlled study of the efficacy of 4 weeks of supplementation with ω‐3 polyunsaturated fatty acids in cases of unexplained oligohydramnios. Journal of Perinatology 2016;36(11):944‐7. - PubMed
Jamilian 2016 {published data only}
-
- Asemi Z, IRCT201406305623N20. Effect of omega‐3 supplementation on inflammatory factors, biomarkers of oxidative stress and pregnancy outcomes in gestational diabetes. en.search.irct.ir/view/18933 (first received 16 July 2014).
-
- Jamilian M, Samimi M, Kolahdooz F, Khalaji F, Razavi M, Asemi Z. Omega‐3 fatty acid supplementation affects pregnancy outcomes in gestational diabetes: a randomized, double‐blind, placebo‐controlled trial. Journal of Maternal‐Fetal & Neonatal Medicine 2016;29(4):669‐75. - PubMed
Jamilian 2017 {published data only}
-
- Jamilian M, Samimi M, Afshar Ebrahimi F, Hashemi T, Taghizadeh M, Sanami M, et al. The effects of vitamin D and omega‐3 fatty acid co‐supplementation on glycemic control and lipid concentrations in patients with gestational diabetes. Journal of Clinical Lipidology 2017;11(2):459‐68. - PubMed
Judge 2007 {published data only}
-
- Courville AB, Harel O, Lammi‐Keefe CJ. Consumption of a DHA‐containing functional food during pregnancy is associated with lower infant ponderal index and cord plasma insulin concentration. British Journal of Nutrition 2011;106(2):208‐12. - PubMed
-
- Courville AB, Keplinger MR, Judge MP, Lammi‐Keefe CJ. Plasma or red blood cell phospholipids can be used to assess docosahexaenoic acid status in women during pregnancy. Nutrition Research 2009;29:151‐5. - PubMed
-
- Durham H, Wood JT, Williams JS, Geaghan JP, Makriyannis A, Lammi‐Keefe CJ. How do plasma endocannabinoids and inflammatory markers respond to docosahexaenoic acid (DHA) supplementation in pregnancy?. FASEB Journal 2011;25:777.4.
-
- Judge MP. Impact of maternal docosahexaenoic acid (DHA) supplementation in the form of a functional food during pregnancy on infant neurodevelopment: A comparison of vision, memory, temperament and problem‐solving abilities. Proquest Dissertations Publishing, 2006.
-
- Judge MP, Cong X, Harel O, Courville AB, Lammi‐Keefe CJ. Maternal consumption of a DHA‐containing functional food benefits infant sleep patterning: An early neurodevelopmental measure. Early Human Development 2012;88(7):531‐7. - PubMed
Judge 2014 {published data only}
-
- Judge MP, Beck C, Durham H, Lammi‐Keefe C, McKelvey MM. Specific symptoms of postpartum depression (PPD) are decreased in mothers supplemented with docosahexaenoic acid (DHA, 22:6n‐3) during pregnancy. Nursing Research 2013;62(2):E21.
-
- Judge MP, Beck CT, Durham H, McKelvey MM, Lammi‐Keefe CJ. Maternal docosahexaenoic acid (DHA, 22:6n‐3) consumption during pregnancy decreases postpartum depression (PPD) symptomatology. FASEB Journal 2011;25:349.7.
-
- Judge MP, Beck CT, Durham H, McKelvey MM, Lammi‐Keefe CJ. Pilot trial evaluating maternal docosahexanoic acid consumption during pregnancy: decreased postpartum depressive symptomatology. International Journal of Nursing Sciences 2014;1:339‐45.
Kaviani 2014 {published data only}
-
- Azima S. The effect of omega‐3 fatty acid capsules on anxiety and quality of life gravida during pregnancy depression that refer to health clinic in Shiraz, Iran. en.search.irct.ir/view/11938 (first received 3 January 2013).
Keenan 2014 {published data only}
-
- Keenan K, NCT01158976. Impact of omega‐3 intake during pregnancy on maternal stress and infant outcome. clinicaltrials.gov/show/NCT01158976 (first received 8 July 2010).
Khalili 2016 {published data only}
-
- Faraji I, Ostadrahimi A, Farshbaf‐Khalili A, Aslani H. The impact of supplementation with fish oil on lipid profile of pregnant mothers: a randomized controlled trial. Crescent Journal of Medical and Biological Sciences 2016;3(3):100‐6.
-
- Farshbaf‐Khalili A, IRCT2013100914957N1. The effect of fish‐oil supplementation on pregnancy outcomes in mother and infant: a randomized controlled trial. en.search.irct.ir/view/15365 (first received 14 February 2014).
-
- Farshbaf‐Khalili A, Mohammad‐Alizadeh S, Mohammadi F, Ostadrahimi A. Fish‐oil supplementation and maternal mental health: a triple‐blind, randomized controlled trial. Iranian Red Crescent Medical Journal 2017;19(1):e36237. [IRCT2013100914957N1]
-
- Khalili AF, Mohamad‐Alizadeh S, Darabi M, Hematzadeh S, Mehdizadeh A, Shaaker M, et al. The effect of fish oil supplementation on serum phospholipid fatty acids profile during pregnancy: a double blind randomized controlled trial. Women & Health 2017;57(2):137‐53. [DOI: 10.1080/03630242.2016.1159269] - DOI - PubMed
-
- Ostadrahimi A, Mohammad‐Alizadeh S, Mirghafourvand M, Farshbaf‐Khalili S, Jafarilar‐Agdam N, Farshbaf‐Khalili A. The effect of fish oil supplementation on maternal and neonatal outcomes: a triple‐blind, randomized controlled trial. Journal of Perinatal Medicine 2017;45(9):1069‐77. [DOI: 10.1515/jpm-2016-0037] - DOI - PubMed
Knudsen 2006 {published data only}
-
- Knudsen VK, Hansen HS, Osterdal ML, Mikkelsen TB, Mu H, Olsen SF. Fish oil in various doses or flax oil in pregnancy and timing of spontaneous delivery: a randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2006;113(5):536‐43. - PubMed
Krauss‐Etschmann 2007 {published data only}
-
- Broekaert I, Campoy C, Iznaola C, Hoffman B, Mueller‐Felber W, Koletzko BV. Visual evoked potentials in infants after dietary supply of docosahexaenoic acid and 5‐methyl‐tetrahydrofolate during pregnancy. Journal of Pediatric Gastroenterology and Nutrition 2004;39(Suppl 1):S33.
-
- Campoy C, Escolano‐Margarit MV, Ramos R, Parrilla‐Roure M, Csabi G, Beyer J, et al. Effects of prenatal fish‐oil and 5‐methyltetrahydrofolate supplementation on cognitive development of children at 6.5 y of age. American Journal of Clinical Nutrition 2011;94(6 Suppl):1880S‐8S. - PubMed
-
- Campoy C, Marchal G, Decsi T, Cruz M, Szabo E, Demmelmair H, et al. Spanish pregnant women's plasma phospholipids LC‐PUFAs concentrations and its influence on their newborns. Journal of Pediatric Gastroenterology and Nutrition 2004;39(Suppl 1):S11.
-
- Catena A, Martinez‐Zaldivar C, Diaz‐Piedra C, Torres‐Espinola FJ, Brandi P, Perez‐Garcia M, et al. On the relationship between head circumference, brain size, prenatal long‐chain PUFA/5‐methyltetrahydrofolate supplementation and cognitive abilities during childhood. British Journal of Nutrition 2017 [Epub ahead of print]:1‐9. - PubMed
-
- Catena A, Muñoz‐Machicao JA, Torres‐Espínola FJ, Martínez‐Zaldívar C, Diaz‐Piedra C, Gil A, et al. Folate and long‐chain polyunsaturated fatty acid supplementation during pregnancy has long‐term effects on the attention system of 8.5‐y‐old offspring: a randomized controlled trial. American Journal of Clinical Nutrition 2016;103(1):115‐27. - PubMed
Krummel 2016 {published data only}
-
- Escaname EN, Foster BA, Larsen B, Siddiqui SK, Menchaca J, Hale D, et al. Randomized controlled trial of DHA supplementation in pregnancy: results from long term follow up of offspring. Pediatric Academic Societies Annual Meeting; 2016 April 30‐May 3; Baltimore (MD). 2016:3365.8.
-
- Galindo M, Gaccioli F, Lager S, Ramirez V, Meireles C, Miller E, et al. Omega‐3 supplementation in obese pregnant women reduces placental mTOR activation. Reproductive Sciences 2012;19(3 Suppl):113A.
-
- Krummel DA. DHA supplements to improve insulin sensitivity in obese pregnant women (the omega‐3 pregnancy study). clinicaltrials.gov/ct2/show/NCT00865683 (first received 19 March 2009). [NCT00865683]
-
- Krummel DA, Kuhn AB, Cassin AM, Davis SL, Walker LE, Khoury JC, et al. Effect of docosahexaenoic acid supplementation on glucose tolerance and markers of inflammation in overweight/obese pregnant women: a double‐blind, randomized, controlled trial. Journal of Pregnancy and Child Health 2016;3:212.
Laivuori 1993 {published data only}
-
- Laivuori H, Hovatta O, Viinikka L, Ylikorkala O. Dietary supplementation with primrose oil or fish oil does not change urinary excretion of prostacyclin and thromboxane metabolites in pre‐eclamptic women. Prostaglandins Leukotrienes and Essential Fatty Acids 1993;49:691‐4. - PubMed
Makrides 2010 {published data only}
-
- Ahmed S, Makrides M, Sim N, McPhee A, Quinlivan J, Gibson R, et al. Analysis of hospital cost outcome of DHA‐rich fish‐oil supplementation in pregnancy: evidence from a randomized controlled trial. Prostaglandins, Leukotrienes, and Essential Fatty Acids 2015;102‐103:5‐11. - PubMed
-
- Best K, ACTRN12615000498594. Six year follow up of a randomised controlled trial to compare maternal omega‐3 long chain poly‐unsaturated fatty acids (n‐3 LCPUFA) supplementation versus vegetable oil in pregnancy, on allergic disease outcomes in the school age child. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=367939 (first received 24 April 2015). [ACTRN12615000498594]
-
- Best K, Sullivan T, Gold M, Kennedy D, Martin J, Palmer D, et al. Six‐year follow up of children at high hereditary risk of allergy, born to mothers supplemented with docosahexaenoic acid (DHA) in the DOMInO trial. Journal of Paediatrics and Child Health 2015;51(Suppl 1):58.
-
- Best KP, Sullivan T, Palmer D, Gold M, Kennedy DJ, Martin J, et al. Prenatal fish oil supplementation and allergy: 6‐year follow‐up of a randomized controlled trial. Pediatrics 2016;137(6):e20154443. - PubMed
-
- Best KP, Sullivan TR, Palmer DJ, Gold M, Martin J, Kennedy D, et al. Prenatal omega‐3 LCPUFA and symptoms of allergic disease and sensitization throughout early childhood ‐ a longitudinal analysis of long‐term follow‐up of a randomized controlled trial. World Allergy Organization Journal 2018;11(1):10. - PMC - PubMed
Malcolm 2003 {published data only}
-
- Malcolm CA, Hamilton R, McCulloch DL, Montgomery C, Weaver LT. Scotopic electroretinogram in term infants born of mothers supplemented with docosahexaenoic acid during pregnancy. Investigative Ophthalmology & Visual Science 2003;44:3685‐91. - PubMed
-
- Malcolm CA, McCulloch DL, Montgomery C, Shepherd A, Weaver LT. Maternal docosahexaenoic acid supplementation during pregnancy and visual evoked potential development in term infants: a double blind, prospective, randomised trial. Archives of Diseases in Childhood Fetal & Neonatal Edition 2003;90:F383‐90. - PMC - PubMed
-
- McCulloch D, Malcolm CA, Montgomery C, Hamilton RH, Weaver LT. Maternal fish oil supplementation and visual maturation in term infants. Optometry and Vision Science 2002;79(12):296.
-
- Montgomery C, Speake BK, Cameron A, Sattar N, Weaver LT. Maternal docosahexaenoic acid supplementation and fetal accretion. British Journal of Nutrition 2003;90:135‐45. - PubMed
Mardones 2008 {published data only}
-
- Mardones F, Urrutia MT, Villarroel L, Rioseco A, Castillo O, Rozowski J, et al. Effects of a dairy product fortified with multiple micronutrients and omega‐3 fatty acids on birth weight and gestation duration in pregnant Chilean women. Public Health Nutrition 2008;11(1):30‐40. - PubMed
Martin‐Alvarez 2012 {published data only}
-
- Martin Alvarez E, Pena‐Caballero M, Hurtado‐Suazo JA, Kajarabille N, Lara‐Villoslada F, Ochoa JJ. Variability in adipokines profile of newborns and their mothers after DHA supplementation in pregnancy [PS‐053]. Archives Disease in Childhood 2014;99(Suppl 2):A131.
-
- Martin E, Pena M, Kajarabille N, Hurtado JA, Lara‐Villoslada F, Ochoa JJ. Effect of DHA supplementation during pregnancy and lactation on several adipokines in pregnant women and their neonates. Journal of Maternal‐Fetal & Neonatal Medicine 2014;27(Suppl 1):85.
-
- Martin‐Alvarez E, Guerrero‐Montenegro B, Romero‐Paniagua MT, Lara‐Villoslada F, Hurtado‐Suazo JA. Maternal docosahexaenoic acid supplementation during pregnancy and lactation can modulate oxidative stress in the term newborn. Journal of Maternal‐Fetal and Neonatal Medicine 2012;25(S2):40.
Miller 2016 {published data only}
-
- Harris M, Miller S, Baker S, McGirr K, Davalos D. The omega smart baby project; effect of maternal DHA on infant development. FASEB Journal 2014;28(1 Suppl):[abstract no: 269.1].
-
- Miller SM, Harris MA, Baker SS, Davalos DB, Clark AM, McGirr KA. Intake of total omega‐3 docosahexaenoic acid associated with increased gestational length and improved cognitive performance at 1 year of age. Journal of Nutritional Health & Food Engineering 2016;5(3):00176.
Min 2014 {published data only}
-
- Min Y, Djahanbakhch O, Hutchinson J, Bhullar AS, Raveendran M, Hallot A, et al. Effect of docosahexaenoic acid‐enriched fish oil supplementation in pregnant women with type 2 diabetes on membrane fatty acids and fetal body composition ‐ double‐blinded randomized placebo‐controlled trial. Diabetic Medicine 2014;31:1331‐40. - PubMed
-
- Min Y, ISRCTN68997518. Dietary omega‐3 and omega‐6 fatty acids supplementation in pregnant women with diabetes: a randomised, double‐blind, placebo‐controlled trial. isrctn.com/ISRCTN68997518 (first received 30 July 2010).
Min 2014 [diabetic women] {published data only}
-
- Min Y, Djahanbakhch O, Hutchinson J, Bhullar AS, Raveendran M, Hallot A, et al. Effect of docosahexaenoic acid‐enriched fish oil supplementation in pregnant women with type 2 diabetes on membrane fatty acids and fetal body composition ‐ double‐blinded randomized placebo‐controlled trial. Diabetic Medicine 2014;31:1331‐40. - PubMed
-
- Min Y, ISRCTN68997518. Dietary omega‐3 and omega‐6 fatty acids supplementation in pregnant women with diabetes: a randomised, double‐blind, placebo‐controlled trial. isrctn.com/ISRCTN68997518 (first received 30 July 2010).
Min 2016 {published data only}
-
- Min Y, Djahanbakhch O, Hutchinson J, Eram S, Bhullar AS, Namugere I, et al. Efficacy of docosahexaenoic acid‐enriched formula to enhance maternal and fetal blood docosahexaenoic acid levels: randomized double‐blinded placebo‐controlled trial of pregnant women with gestational diabetes mellitus. Clinical Nutrition 2016;35:608‐14. - PubMed
Mozurkewich 2013 {published data only}
-
- Berman D, Limb R, Somers E, Clinton C, Romero V, Mozurkewich E. Prenatal omega‐3 supplementation and risk of eczema among offspring at age 36 months: long‐term follow‐up of the mothers, omega‐3, & mental health trial. American Journal of Obstetrics and Gynecology 2015;212(Suppl 1):S162.
-
- Brown ST, Tyner J, Mozurkewich EL, Clinton CM, Schrader R, Marcus S, et al. Fatty acid ratios and depressive symptoms in pregnancy: a secondary analysis of the Mothers, Omega‐3 & Mental Health Study. Reproductive Sciences 2013;20(3 Suppl):92A‐3A.
-
- Jain JA, Tyner JE, Holbrook BD, Williams JA, Mozurkewich EL. Is vitamin D associated with insulin sensitivity in maternal or umbilical cord blood?. Reproductive Sciences 2015;22(Suppl 1):251A.
-
- Mozurkewich E. Does EPA or DHA prevent depressive symptoms in pregnancy and postpartum?. clinicaltrials.gov/ct2/show/record/NCT00711971 (first received 9 July 2008). [NCT00711971]
Mulder 2014 {published data only}
-
- Friesen RW, Innis SM. Dietary arachidonic acid to EPA and DHA balance is increased among Canadian pregnant women with low fish intake. Journal of Nutrition 2009;139(12):2344‐50. - PubMed
-
- Friesen RW, Innis SM. Linoleic acid is associated with lower long‐chain n‐6 and n‐3 fatty acids in red blood cell lipids of Canadian pregnant women. American Journal of Clinical Nutrition 2010;91(1):23‐31. - PubMed
-
- Innis SM. N‐3 fatty acid requirements for human development. clinicaltrials.gov/ct2/show/NCT00620672 (first received 21 February 2008).
-
- Innis SM, Friesen RW. Essential N‐3 fatty acids in pregnant women and early visual acuity maturation in term infants. American Journal of Clinical Nutrition 2008;87(3):548‐57. - PubMed
-
- Innis SM, Friesen RW. Maternal DHA supplementation in pregnancy: a double blind randomized prospective trial of maternal N‐3 fatty acid status, human milk fatty acids and infant development. Pediatric Academic Societies Annual Meeting; 2007 May 5‐8; Toronto (Canada). 2007.
Noakes 2012 {published data only}
-
- Anonymous. Salmon in pregnancy study (SiPS). clinicaltrials.gov/ct2/show/NCT00801502 (first received 3 December 2008).
-
- Garcia‐Rodriguez C, Helmersson‐Karlqvis J, Mesa M, Miles E, Noakes P, Vlachava M, et al. Increased intake of salmon decreases F2‐isoprostanes in pregnant women. Annals of Nutrition and Metabolism 2011;58(Suppl 3):122‐3.
-
- Garcia‐Rodriguez C, Mesa M, Olza J, Vlachava M, Kremmyda L, Diaper N, et al. Farmed salmon supplementation enhances the enzymatic defence system. Annals of Nutrition and Metabolism 2011;58(Suppl 3):89‐90.
-
- Garcia‐Rodriguez CE, Helmersson‐Karlqvist J, Mesa MD, Miles EA, Noakes PS, Vlachava M, et al. Does increased intake of salmon increase markers of oxidative stress in pregnant women? The salmon in pregnancy study. Antioxidants & Redox Signaling 2011;15(11):2819‐23. - PubMed
-
- Garcia‐Rodriguez CE, Mesa MD, Olza J, Vlachava M, Kremmyda LS, Diaper ND, et al. Does consumption of two portions of salmon per week enhance the antioxidant defense system in pregnant women?. Antioxidants and Redox Signaling 2012;16(12):1401‐6. - PubMed
Ogundipe 2016 {published data only}
-
- Ogundipe E. Maternal fish oil supplementation in high risk pregnancies and the effect on the infants' brain MRI findings and neurobehavioural outcomes. isrctn.com/ISRCTN24068733 (first received 13 June 2011). [ISRCTN24068733]
-
- Ogundipe E, Ghaus K, Wang Y, Talbot I, Johnson MR, Crawford M. FOSS Trial: antenatal maternal DHA and AA lipids and their psycho‐behavioural status. Journal of Maternal‐Fetal & Neonatal Medicine 2014;27(Suppl 1):202.
-
- Ogundipe E, Tusor N, Wang Y, Johnson MR, Edwards AD, Crawford MA. Randomized controlled trial of brain specific fatty acid supplementation in pregnant women increases brain volumes on MRI scans of their newborn infants. Prostaglandins, Leukotrienes and Essential Fatty Acids 2018;138:6‐13. - PubMed
-
- Ogundipe E, Wang Y, Johnson MR, Crawford MA. Monounsaturated fatty acids in early pregnancy and preterm birth. Journal of Maternal‐Fetal and Neonatal Medicine 2016;29(Suppl 1):264.
Oken 2013 {published data only}
Olsen 1992 {published data only}
-
- Dalby Sorensen J, Olsen SF, Pedersen AK, Boris J, Secher NJ, FitzGerald GA. Effect of fish oil supplementation in the third trimester of pregnancy on prostacyclin and thromboxane production. American Journal of Obstetrics and Gynecology 1993;168:915‐22. - PubMed
-
- Dalby Sorensen J, Secher NJ, Olsen SF. Effects of n‐3 fatty acids on blood pressure in the third trimester of pregnancy. 7th World Congress of Hypertension in Pregnancy; 1990; Perugia (Italy). 1990:343.
-
- Hansen S, Strom M, Maslova E, Dahl R, Hoffmann HJ, Rytter D, et al. Fish oil supplementation during pregnancy and allergic respiratory disease in the adult offspring. Journal of Allergy and Clinical Immunology 2017;139(1):104‐11. - PubMed
-
- Olsen S, NCT01353807. Impact of fish oil supplementation in 3rd trimester of pregnancy on maternal and offspring health. clinicaltrials.gov/show/NCT01353807 (first received 16 May 2011). [NCT01353807]
-
- Olsen SF, Dalby Sorensen J, Secher NJ, Hedegaard M, Brink Henriksen T, Hansen HS, et al. Randomised controlled trial of effect of fish‐oil supplementation on pregnancy duration. Lancet 1992;339:1003‐7. - PubMed
Olsen 2000 {published data only}
-
- Hansen S, Maslova E, Strom M, Secher NJ, Olsen SF. Does mode of delivery modify the association between intake of fish oil during pregnancy and the risk of child asthma? Results from a randomized controlled trial with twin pregnancies. Acta Obstetricia et Gynecologica Scandinavica 2013;92:17.
-
- Olsen S, NCT02229526. Randomised clinical trials of fish oil supplementation in high risk pregnancies. clinicaltrials.gov/show/NCT02229526 (first received 1 September 2014). [NCT02229526]
-
- Olsen SF, Osterdal ML, Salvig JD, Weber T, Tabor A, Secher NJ. Duration of pregnancy in relation to fish oil supplementation and habitual fish intake: a randomised clinical trial with fish oil. European Journal of Clinical Nutrition 2007;61(8):976‐85. - PubMed
-
- Olsen SF, Secher NJ, Tabor A, Weber T, Walker JJ, Gluud C. Randomised clinical trials of fish oil supplementation in high risk pregnancies. British Journal of Obstetrics and Gynaecology 2000;107:382‐95. - PubMed
-
- Secher NJ, Sorensen JD. Biochemical and epidemiological evidence for the preventive effect of fish oil on pre‐eclampsia. 9th International Congress of the International Society for the Study of Hypertension in Pregnancy; 1994 March 15‐18; Sydney (Australia). 1994:77.
Olsen 2000 [twins] {published data only}
-
- Hansen S, Maslova E, Strom M, Secher NJ, Olsen SF. Does mode of delivery modify the association between intake of fish oil during pregnancy and the risk of child asthma? Results from a randomized controlled trial with twin pregnancies. Acta Obstetricia et Gynecologica Scandinavica 2013;92:17.
-
- Olsen S, NCT02229526. Randomised clinical trials of fish oil supplementation in high risk pregnancies. clinicaltrials.gov/show/NCT02229526 (first received 1 September 2014). [NCT02229526]
-
- Olsen SF, Osterdal ML, Salvig JD, Weber T, Tabor A, Secher NJ. Duration of pregnancy in relation to fish oil supplementation and habitual fish intake: a randomised clinical trial with fish oil. European Journal of Clinical Nutrition 2007;61(8):976‐85. - PubMed
-
- Olsen SF, Secher NJ, Tabor A, Weber T, Walker JJ, Gluud C. Randomised clinical trials of fish oil supplementation in high risk pregnancies. British Journal of Obstetrics and Gynaecology 2000;107:382‐95. - PubMed
-
- Secher NJ, Sorensen JD. Biochemical and epidemiological evidence for the preventive effect of fish oil on pre‐eclampsia. 9th International Congress of the International Society for the Study of Hypertension in Pregnancy; 1994 March 15‐18; Sydney (Australia). 1994:77.
Onwude 1995 {published data only}
-
- Onwude J, Hjartar H, Tuffnell D, Thornton JG, Lilford RJ. Fish oil in high risk pregnancy: a randomised, double blind, placebo‐controlled trial. Proceedings of 26th British Congress of Obstetrics and Gynaecology; 1992 July 7‐10; Manchester (UK). 1992:430.
-
- Onwude JL, Lilford RJ, Hjartardottir H, Staines A, Tuffnell D. A randomised double blind placebo controlled trial of fish oil in high risk pregnancy. British Journal of Obstetrics and Gynaecology 1995;102:95‐100. - PubMed
Otto 2000 {published data only}
-
- Otto SJ, Houwelingen AC, Hornstra G. The effect of supplementation with docosahexaenoic and arachidonic acid derived from single cell oils on plasma and erythrocyte fatty acids of pregnant women in the second trimester. Prostaglandins Leukotrienes, and Essential Fatty Acids 2000;63:323‐8. - PubMed
Pietrantoni 2014 {published data only}
Ramakrishnan 2010 {published data only}
-
- Barraza‐Villarreal A, Escamilla‐Nunez MC, Hernandez‐Cadena L, Navarro‐Olivos E, Texcalac‐Sangrador JL, Shackleton C, et al. Volatile organic compounds air concentrations and respiratory function in Mexican preschoolers from mothers whose participated in a randomized clinical trial during pregnancy. American Journal of Respiratory Critical Care 2012;185:A6887.
-
- Gonzalez‐Casanova I, Rzehak P, Hao W, Aryeh S, Barraza‐Villarreal A, Garcia‐Feregrino R, et al. Fatty acid desaturase single nucleotide polymorphisms modify the effect of prenatal supplementation with docosahexaenoic acid on birth weight. FASEB Journal 2015;29(1 Suppl):403.3.
-
- Gonzalez‐Casanova I, Rzehak P, Stein AD, Garcia Feregrino R, Rivera Dommarco JA, Barraza‐Villareal A, et al. Maternal single nucleotide polymorphisms in the fatty acid desaturase 1 and 2 coding regions modify the impact of prenatal supplementation with DHA on birth weight. American Journal of Clinical Nutrition 2016;103(4):1171‐8. - PMC - PubMed
-
- Gonzalez‐Casanova I, Stein A, Hao W, Feregrino R, Romieu I, Barraza‐Villarreal A, et al. Height and BMI at five years of age following prenatal supplementation with docosahexaenoic acid in Mexico. FASEB Journal 2014;28(1 Suppl 1):256.8.
Ranjkesh 2011 {published data only}
-
- Lalooha F, Ghaleh TD, Pakniiat H, Ranjkesh F, Gholshahi T, Mashrabi O. Evaluation of the effect of omega‐3 supplements in the prevention of preeclampsia among high risk women. African Journal of Pharmacy and Pharmacology 2012;6(35):2580‐3. [DOI: 10.5897/AJPP11.836] - DOI
-
- Ranjkesh F. The effect of omega3 supplementation in preeclampsia in pregnant women at risk in Qazvin [IRCT138706061113N1]. en.irct.ir/trial/145 (first received 27 March 2010).
-
- Ranjkesh F, Laluha F, Pakniat H, Kazemi H, Golshahi T, Esmaili S. Effect of omeg‐3 supplementation on preeclampsia in high risk pregnant women. Journal of Qazvin University of Medical Sciences 2011;15(2):28‐33.
Razavi 2017 {published data only}
-
- Razavi M. Clinical trial of the effect of combined omega‐3 fatty acids and vitamin D supplementation compared with the placebo on metabolic profiles and pregnancy outcomes in patients with gestational diabetes. en.search.irct.ir/view/35656 (first received 9 February 2017). [IRCT201701305623N106]
-
- Razavi M, Jamilian M, Samimi M, Afshar Ebrahimi F, Taghizadeh M, Bekhradi R, et al. The effects of vitamin D and omega‐3 fatty acids co‐supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in patients with gestational diabetes. Nutrition & Metabolism 2017;14:80. [DOI: 10.1186/s12986-017-0236-9] - DOI - PMC - PubMed
Razavi 2017 [vit D] {published data only}
-
- Razavi M. Clinical trial of the effect of combined omega‐3 fatty acids and vitamin D supplementation compared with the placebo on metabolic profiles and pregnancy outcomes in patients with gestational diabetes. en.search.irct.ir/view/35656 (first received 9 February 2017). [IRCT201701305623N106]
-
- Razavi M, Jamilian M, Samimi M, Afshar Ebrahimi F, Taghizadeh M, Bekhradi R, et al. The effects of vitamin D and omega‐3 fatty acids co‐supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in patients with gestational diabetes. Nutrition & Metabolism 2017;14:80. [DOI: 10.1186/s12986-017-0236-9] - DOI - PMC - PubMed
Rees 2008 {published data only}
-
- Rees AM, Austin MP, Parker GB. Omega‐3 fatty acids as a treatment for perinatal depression: randomized double‐blind placebo‐controlled trial. Australian and New Zealand Journal of Psychiatry 2008;42:199‐205. - PubMed
Ribeiro 2012 {published data only}
-
- Ribeiro P, Carvalho FD, Abreu Ade A, Sant'anna Mde T, Lima RJ, Carvalho Pde O. Effect of fish oil supplementation in pregnancy on the fatty acid composition of erythrocyte phospholipids and breast milk lipids. International Journal of Food Sciences & Nutrition 2012;63(1):36‐40. - PubMed
Rivas‐Echeverria 2000 {published data only}
-
- Rivas‐Echeverria CA, Echeverria Y, Molina L, Novoa D. Synergic use of aspirin, fish oil and vitamins C and E for the prevention of preeclampsia. Hypertension in Pregnancy 2000;19:30.
Samimi 2015 {published data only}
-
- Samimi M, Jamilian M, Asemi Z, Esmaillzadeh A. Effects of omega‐3 fatty acid supplementation on insulin metabolism and lipid profiles in gestational diabetes: randomized, double‐blind, placebo‐controlled trial. American Journal of Clinical Nutrition 2015;34:388‐93. - PubMed
Sanjurjo 2004 {published data only}
-
- Sanjurjo P, Ruiz‐Sanz JI, Jimeno P, Aldamiz‐Echevarria L, Aquino L, Matorras R, et al. Supplementation with docosahexaenoic acid in the last trimester of pregnancy: maternal‐fetal biochemical findings. Journal of Perinatal Medicine 2004;32(2):132‐6. - PubMed
Smuts 2003a {published data only}
-
- Colombo J, Kannass KN, Shaddy DJ, Kundurthi S, Maikranz JM, Anderson CJ, et al. Maternal DHA and the development of attention in infancy and toddlerhood. Child Development 2004;75(4):1254‐67. - PubMed
-
- Smuts CM, Huang M, Mundy D, Plasse T, Major S, Carlson SE. A randomized trial of docosahexaenoic acid supplementation during the third trimester of pregnancy. Obstetrics & Gynecology 2003;101:469‐79. - PubMed
Smuts 2003b {published data only}
-
- Borod E, Atkinson R, Barclay WR, Carlson SE. Effects of third trimester consumption of eggs high in docosahexaenoic acid on docosahexaenoic acid status and pregnancy. Lipids 1999;34 Suppl:S231. - PubMed
-
- Smuts CM, Borod E, Peeples JM, Carlson SE. High‐DHA eggs: feasibility as a means to enhance circulating DHA in mother and infant. Lipids 2003;38(4):407‐14. - PubMed
Su 2008 {published data only}
-
- Su K‐P, Huang S‐Y, Chiu T‐H, Huang K‐C, Huang C‐L, Chang H‐C, et al. Omega‐3 fatty acids for major depressive disorder during pregnancy: results from a randomized, double‐blind, placebo‐controlled trial. Journal of Clinical Psychiatry 2008;69(4):644‐51. - PubMed
Taghizadeh 2016 {published data only}
-
- Asemi Z, IRCT201507035623N47. Clinical trial of the effect of combined omega‐3 and vitamin E supplementation on pregnancy outcomes in gestational diabetes. en.search.irct.ir/view/24407 (first received 19 July 2015).
-
- Jamilian M, Hashemi Dizaji S, Bahmani F, Taghizadeh M, Memarzadeh MR, Karamali M, et al. A randomized controlled clinical trial investigating the effects of omega‐3 fatty acids and vitamin E co‐supplementation on biomarkers of oxidative stress, inflammation and pregnancy outcomes in gestational diabetes. Canadian Journal of Diabetes 2017;41(2):143‐9. [DOI: 10.1016/j.jcjd.2016.09.004] - DOI - PubMed
-
- Taghizadeh M, Jamilian M, Mazloomi M, Sanami M, Asemi Z. A randomised‐controlled clinical trial investigating the effect of omega‐3 fatty acids and vitamin E co‐supplementation on markers of insulin metabolism and lipid profiles in gestational diabetes. Journal of Clinical Lipidology 2016;10:386‐93. - PubMed
Tofail 2006 {published data only}
-
- Tofail F, Hamadani JD, Ahmed AZ, Mehrin F, Hakim M, Huda SN. The mental development and behavior of low‐birth‐weight Bangladeshi infants from an urban low‐income community. European Journal of Clinical Nutrition 2012;66(2):237‐43. - PubMed
-
- Tofail F, Kabir I, Hamadani JD, Chowdhury F, Yesmin S, Mehreen F, et al. Supplementation of fish‐oil and soy‐oil during pregnancy and psychomotor development of infants. Journal of Health, Population & Nutrition 2006;24(1):48‐56. - PubMed
Valenzuela 2015 {published data only}
Van Goor 2009 {published data only}
-
- Doornbos B, Goor SA, Dijck‐Brouwer DA, Schaafsma A, Kork J, Muskiet FA. Supplementation of a low dose of DHA or DHA+AA does not prevent peripartum depressive symptoms in a small population based sample. Progress in Neuro‐Psychopharmacology & Biological Psychiatry 2009;33(1):49‐52. - PubMed
-
- Goor SA, Dijck‐Brouwer DA, Erwich JJ, Schaafsma A, Mijna Hadders‐Algra M. The influence of supplemental docosahexaenoic and arachidonic acids during pregnancy and lactation on neurodevelopment at eighteen months. Prostaglandins Leukotrienes and Essential Fatty Acids 2011;84(5‐6):139‐46. - PubMed
-
- Goor SA, Dijck‐Brouwer DA, Hadders‐Algra M, Doornbos B, Erwich JJ, Schaafsma A, et al. Human milk arachidonic acid and docosahexaenoic acid contents increase following supplementation during pregnancy and lactation. Prostaglandins Leukotrienes, and Essential Fatty Acids 2009;80(1):65‐9. - PubMed
-
- Goor SA, Muskiet FA. The effect of a high DHA‐fish oil and arachidonic acid (AA) supplementation during pregnancy and lactation on LCP status of mother and child and on the neurological development of the baby. trialregister.nl/trialreg/admin/rctview.asp?TC=366 (first received 19 December 2005.
-
- Goor SA, Schaafsma A, Erwich JJ, Dijck‐Brouwer DA, Muskiet FA. Mildly abnormal general movement quality in infants is associated with higher Mead acid and lower arachidonic acid and shows a U‐shaped relation with the DHA/AAratio. Prostaglandins Leukotrienes and Essential Fatty Acids 2010;82:15‐20. - PubMed
Van Winden 2017 {published data only}
-
- Winden KR, Montoro M, Silverstein E, Ovalle B, Shulman I, Ouzounian JG. The use of omega‐3 fatty acids to improve insulin sensitivity in pregnancy: a pilot study of safety and tolerability. Obstetrics and Gynecology 2017;129:174S.
Vaz 2017 {published data only}
References to studies excluded from this review
Escobar 2008 {published data only}
-
- Escobar G, NCT00691418. DHA administration and length of gestation: a feasibility study. clinicaltrials.gov/show/NCT00691418 (first received 5 June 2008). [NCT00691418]
Fievet 1985 {published data only}
-
- Fievet P, Tribout B, Castier B, Dieval J, Capiod JC, Delobel J, et al. Effects of evening primrose oil (EPO) on platelet functions during pregnancy in patients with high risk of toxemia. Kidney International 1985;28:234.
Gholami 2017 {published data only}
-
- Gholami SN, IRCT2015072123269N1. Clinical trial to evaluate the effect of fish oil supplementation on pregnancy outcomes in pregnant women. http://en.search.irct.ir/view/24693 (first received 11 June 2017). [IRCT2015072123269N1]
Herrera 1993 {published data only}
-
- Herrera JA. Nutritional factors and rest reduce pregnancy‐induced hypertension and pre‐eclampsia in positive roll‐over test primigravidas. International Journal of Gynecology & Obstetrics 1993;41:31‐5. - PubMed
Herrera 1998 {published data only}
-
- Herrera JA, Arevalo‐Herrera M, Herrera S. Prevention of preeclampsia by linoleic acid and calcium supplementation: a randomized controlled trial. Obstetrics & Gynecology 1998;91:585‐90. - PubMed
Herrera 2004 {published data only}
-
- Herrera JA, Shahabuddin AK, Ersheng G, Wei Y, Garcia RG, Lopez‐Jaramillo P. Calcium plus linoleic acid therapy for pregnancy‐induced hypertension. International Journal of Gynecology & Obstetrics 2005;91(3):221‐7. - PubMed
-
- Herrera JA, Shahabuddin AK, Faisal M, Ersheng G, Wei Y, Lixia D, et al. Effects of supplementation with oral calcium and linoleic acid in primigravidas at high risk [Efectos de la supplementacion oral con calcio y acido linoleico conjugado en primigravidas de alto riesgo]. Colombia Medica 2004;35(1):31‐7.
Lauritzen 2004 {published data only}
-
- Asserhoj M, Nehammer S, Matthiessen J, Michaelsen KF, Lauritzen L. Maternal fish oil supplementation during lactation may adversely affect long‐term blood pressure, energy intake, and physical activity of 7‐year‐old boys. Journal of Nutrition 2009;139(2):298‐304. - PubMed
-
- Cheatham CL, Nerhammer AS, Asserhoj M, Michaelsen KF, Lauritzen L. Fish oil supplementation during lactation: effects on cognition and behavior at 7 years of age. Lipids 2011;46(7):637‐45. - PubMed
-
- Cheatham CL, Nerhammer S, Asserhoj M, Michaelsen KF, Lauritzen L. Fish oil supplementation during lactation: effects on cognition and behavior at 7 years of age. FASEB Journal 2011;25(Suppl):766.9. - PubMed
-
- Larnkjaer A, Christensen JH, Michaelsen KF, Lauritzen L. Maternal fish oil supplementation during lactation does not affect blood pressure, pulse wave velocity, or heart rate variability in 2.5‐y‐old children. Journal of Nutrition 2006;136(6):1539‐44. - PubMed
-
- Lauritzen L, Halkjaer LB, Mikkelsen TB, Olsen SF, Michaelsen KF, Loland L, et al. Fatty acid composition of human milk in atopic Danish mothers. American Journal of Clinical Nutrition 2006;84(1):190‐6. - PubMed
Marangell 2004 {published data only}
-
- Marangell LB, Martinez JM, Zboyan HA, Chong H, Puryear LJ. Omega‐3 fatty acids for the prevention of postpartum depression: negative data from a preliminary, open‐label pilot study. Depression and Anxiety 2004;19:20‐3. - PubMed
Morrison 1984 {published data only}
-
- Morrison RA, O'Brien PMS, Micklewright A. The effect of dietary supplementation with linoleic acid on the development of pregnancy induced hypertension. 4th World Congress of the International Society for the Study of Hypertension in Pregnancy; 1984 June 18‐21; Amsterdam (The Netherlands). 1984:48.
Morrison 1986 {published data only}
-
- Morrison RA, O'Brien PMS. The effect of dietary supplementation with prostaglandin precursors in pregnancy induced hypertension (PIH). 5th International Congress of the International Society for the Study of Hypertension in Pregnancy; 1986 July 7‐10; Nottingham (UK). 1986.
Nishi 2016 {published data only}
-
- Nishi D, Su KP, Usada, K, Chiang YJ, Guu TW, Haamazaki K, et al. Omega‐3 fatty acid supplementation for expectant mothers with depressive symptoms in Japan and Taiwan: An open‐label trial. Psychiatry and Clinical Neurosciences 2016;70:253‐5. - PubMed
Starling 1990 {published data only}
-
- Starling M, Gunn T, Stewart F. The control of gestational proteinuric hypertension (GPH) with dietary marine lipids. Australian and New Zealand Journal of Medicine 1990;20:357.
Valentine 2013 {published data only}
Velzing‐Aarts 2001 {published data only}
-
- Velzing‐Aarts FV, Klis FR, Dijs FP, Beusekom CM, Landman H, Capello JJ, et al. Effect of three low‐dose fish oil supplements, administered during pregnancy, on neonatal long‐chain polyunsaturated fatty acid status at birth. Prostaglandins, Leukotrienes, and Essential Fatty Acids 2001;65(1):51‐7. [PUBMED: 11487309] - PubMed
Yelland 2016 {published data only}
-
- Yelland LN, Gajewski BJ, Colombo J, Gibson RA, Makrides M, Carlson SE. Predicting the effect of maternal docosahexaenoic acid (DHA) supplementation to reduce early preterm birth in Australia and the United States using results of within country randomized controlled trials. Prostaglandins, Leukotrienes, and Essential Fatty Acids 2016;112:44‐9. - PMC - PubMed
References to studies awaiting assessment
Farahani 2010 {published data only}
-
- Farahani MD. A clinical trial on effect of omega‐3 fatty acids on pregnancy outcome. en.search.irct.ir/view/2314 (first received 3 June 2010). [IRCT138811173291N1]
Gopalan 2004 {published data only}
-
- Gopalan S, Patnaik R, Ganesh K. Feasible strategies to combat low birth weight and intra‐uterine growth retardation. Journal of Pediatric Gastroenterology and Nutrition 2004;39(Suppl 1):S37.
Jamilian 2018 {published data only}
-
- Jamilian M, Samimi M, Mirhosseini N, Afshar Ebrahimi F, Aghadavod E, Taghizadeh M, et al. A randomized double‐blinded, placebo‐controlled trial investigating the effect of fish oil supplementation on gene expression related to insulin action, blood lipids, and inflammation in gestational diabetes mellitus‐fish oil supplementation and gestational diabetes. Nutrients 2018;10(2):E163. - PMC - PubMed
Kadiwala 2015 {published data only}
-
- Kadiwala SM, Ramirez V, Miller E, Matula K, Sifford S, Hakala K, et al. Impact of maternal docosahexaenoic acid supplementation on toddler growth and body composition. Journal of Investigative Medicine 2015;63(2):474.
Laitinen 2013 {published data only}
-
- Laitinen K. Nutrition and pregnancy intervention study. clinicaltrials.gov/ct2/show/NCT01922791 (first received 12 August 2013). [NCT01922791]
-
- Mokkala K, Pussinen P, Houttu N, Koivuniemi E, Vahlberg T, Laitinen K. The impact of probiotics and n‐3 long‐chain polyunsaturated fatty acids on intestinal permeability in pregnancy: a randomised clinical trial. Beneficial Microbes 2018;9(2):199‐208. - PubMed
Lazzarin 2009 {published data only}
-
- Lazzarin N, Vaquero E, Exacoustos C, Bertonotti E, Romanini ME, Arduini D. Low‐dose aspirin and omega‐3 fatty acids improve uterine artery blood flow velocity in women with recurrent miscarriage due to impaired uterine perfusion. Fertility and Sterility 2009;92(1):296‐300. - PubMed
Parisi 2013 {published data only}
-
- Parisi F, Brunetti M, Berti C, Capriata IV, Mazzococo MI, Cetin M. Effects of DHS supplementation during pregnancy on fetal body composition. Reproductive Sciences 2013;20(3 Suppl):244A.
Pavlovich 1999 {published data only}
-
- Pavlovich SV, Burlev VA, Vikhliaeva EM. The effect of n‐3 polyunsaturated fatty acids on the lipid spectrum indices in the 2nd‐3rd pregnancy trimesters [Vliianie n‐3 polinenasyshchennykh zhirnykh kislot na pokazateli lipidnogo spektra vo II‐III trimestrakh beremennosti.]. Eksperimentalnaia i Klinicheskaia Farmakologiia 1999;62(6):35‐8. - PubMed
Sajina‐Stritar 1994 {published data only}
-
- Sajina‐Stritar B. Prevention of gestational hypertension and its complications with ASA and N‐3 fatty acids (comparative study). 14th European Congress of Perinatal Medicine; 1994; Helsinki (Finland). 1994:182.
Sajina‐Stritar 1998 {published data only}
-
- Sajina‐Stritar B. The effect of acetylsaliclic acid and N‐3 fatty acids in prevention of complications of hypertension diseases in pregnancy. Zdravstveni Vestnik 1998;67:489‐93.
Salvig 2009 {published data only}
-
- Salvig J, Hjort J, Moeller M, Holmskov A, Weber T, Olsen S, et al. Randomised clinical trial of fish oil for prevention of preterm birth in high risk women. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S529.
Salzano 2001 {published data only}
-
- Salzano P, Felicetti M, Laboccetta A, Borrelli P, Domenico A, Borrelli A. Prevention of gestational hypertension with calcium, linoleic acid, mono and polyunsaturated fatty acid supplements. Minerva Ginecologica 2001;53(4):235‐8. - PubMed
Stoutjesdijk 2014 {published data only}
-
- Stoutjesdijk E. Fish oil supplemental dose needed to reach 1 g% DHA+EPA in mature milk (ZOOG MUM). trialregister.nl/trialreg/admin/rctview.asp?TC=4959 (first received 19 November 2014). [NTR4959]
-
- Stoutjesdijk E, Schaafsmab A, Dijck‐Brouwera DA, Muskiet FA. Fish oil supplemental dose needed to reach 1 g% DHA+EPA in mature milk. Prostaglandins, Leukotrienes and Essential Fatty Acids 2018;128:53‐61. - PubMed
Vahedi 2018 {published data only}
-
- Vahedi L, Ostadrahimi A, Edalati‐Fard F, Aslani H, Farshbaf‐Khalili A. Is fish oil supplementation effective on maternal serum FBS, oral glucose tolerance test, hemoglobin and hematocrit in low risk pregnant women? A triple‐blind randomized controlled trial. Journal of Complementary & Integrative Medicine 2018 [Epub ahead of print]. - PubMed
Vakilian 2010 {published data only}
-
- Davod‐Abadi M, Vakilian M, Ranjbar A, Seyed‐Zadeh T. The effect of fish oil in oxidative stress indices in healthy pregnant women. Journal of Shahrekord University of Medical Sciences 2010;11(4 Suppl 1):11.
-
- Vakilian K. The effect of fish oil in oxidative stress indices in healthy pregnant women. en.search.irct.ir/view/2974 (first received 29 May 2010). [IRCT138902091557N3]
Valentine 2014 {published data only}
-
- Valentine CJ, NCT02137408. Docosahexaenoic acid supplementation of women with hypertension in pregnancy to improve endothelial health and reduce the risks of preterm delivery. clinicaltrials.gov/show/NCT02137408 (first received 2014). [NCT02137408]
Valenzuela 2017 {published data only}
-
- Valenzuela Baez RW, Barrera C, Bascunan K, Chamorro R, Valenzuela A. Modification of docosahexaenoic acid composition of milk from women who received DHA from a milk formula during the pregnancy and breastfeeding period. Annals of Nutrition and Metabolism 2017;71(Suppl 2):506, Abstract no: 144‐766.
References to ongoing studies
Albert 2017 {published data only}
-
- Albert B. Omega‐3 supplementation during pregnancy to improve metabolic health in children of obese mothers. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=373332 (first received 20 July 2017).
Carlson 2017 ADORE {published data only}
Carvajal 2014 {published data only}
-
- Carvajal J. Docosahexaenoic acid (DHA) supplementation during pregnancy to prevent deep placentation disorders: a randomized clinical trial and a study of the molecular pathways of abnormal placentation prevention. clinicaltrials.gov/show/NCT02336243 (first received 12 January 2015). [NCT02336243]
de Carvalho 2017 {published data only}
-
- Carvalho Sardinha FL. Impact of fish oil or probiotic intake on maternal obesity and molecular biomarkers in the placenta. clinicaltrials.gov/ct2/show/record/NCT03215784 (first received 12 July 2017). [NCT03215784]
Dos Santos 2018 {published data only}
-
- Dos Santos LC. Omega‐3 supplementation during pregnancy to prevent postpartum depressive symptoms and possible effect on breastfeeding, child growth and development. ensaiosclinicos.gov.br/rg/RBR‐6gbzw6/ (first received 16 June 2018).
Dragan 2013 {published data only}
-
- Dragan S, ISRCTN36705743. The impact of EPA and DHA supplementation on the content of lipids in the pregnant women and the fetus. isrctn.com/ISRCTN36705743 (first received 25 October 2016). [ISRCTN36705743]
FOPCHIN {unpublished data only}
-
- NCT02770456. Fish oil supplementation to prevent preterm delivery in China: a randomized controlled trial. clinicaltrials.gov/ct2/show/NCT02770456 (first received 11 May 2016). [NCT02770456]
Garg 2017 {published data only}
-
- Garg M, ACTRN12617000177358. Omega‐3 fish oil for the prevention of gestational diabetes: a double‐blind, randomized controlled proof of concept study. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=371580 (first received 30 January 2017).
Garmendia 2015 {published data only}
-
- Garmendia M, NCT02574767. Diet and physical activity counselling and n3‐long chain (PUFA) supplementation in obese pregnant women (MIGHT). clinicaltrials.gov/ct2/show/NCT02574767 (first received 7 October 2015). [NCT02574767]
-
- Garmendia ML, Corvalan C, Casanello P, Araya M, Flores M, Bravo A, et al. Effectiveness on maternal and offspring metabolic control of a home‐based dietary counseling intervention and DHA supplementation in obese/overweight pregnant women (might study): a randomized controlled trial‐study protocol. Contemporary Clinical Trials 2018;70:35‐40. - PubMed
Ghebremeskel 2014 {published data only}
-
- Ghebremeskel K, ISRCTN03848493. DHA for PREGnant women: is the current recommendation appropriate for women with very low intake and status?. isrctn.com/ISRCTN03848493 (first received 16 May 2014). [ISRCTN03848493]
Hegarty 2012 {published data only}
-
- Hegarty B, ACTRN12612000405819. Long‐chain omega‐3 fatty acids for mood stabilization during pregnancy in women with bipolar disorder ‐ a randomized controlled trial. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12612000405819 (first received 2012). [ACTRN12612000405819]
Hendler 2017 {published data only}
-
- Hendler I. The effect of alpha linolenic acid (ALA) supplementation on essential fatty acids profile during pregnancy compared to common supplements and the epigenetic effect on the newborn. clinicaltrials.gov/show/NCT03040856 (first received 2017). [NCT03040856]
Khandelwal 2012 {published data only}
-
- Khandelwal S. Effect of docosa‐hexaenoic acid (DHA) supplementation during pregnancy on newborn outcomes in India ‐ the DHANI randomized controlled trial. clinicaltrials.gov/ct2/show/NCT01580345 (first received 19 February 2012). [NCT01580345]
Kodkhany 2017 {published data only}
-
- Kodkhany B, NCT03072277. Maternal docosa‐hexaenoic acid (DHA) supplementation and offspring neurodevelopment in India (DHANI‐2). clinicaltrials.gov/show/NCT03072277 (first received 7 March 2017). [NCT03072277]
Li 2013 {published data only}
-
- Li D, NCT01912170. Effect of omega‐3 fatty acids on insulin sensitivity in Chinese gestational diabetic patients. clinicaltrials.gov/ct2/show/NCT01912170 (first received 27 July 2013). [NCT01912170]
Makrides 2013 (ORIP) {published data only}
-
- Makrides M, ACTRN12613001142729. Omega‐3 fats to reduce the incidence of prematurity in healthy women with a singleton or multiple pregnancy less than 20 weeks gestation. anzctr.org.au/ACTRN12613001142729.aspx (first received 27 September 2009). [ACTRN12613001142729]
-
- Zhou J, Best K, Gibson R, McPhee A, Yelland L, Quinlivan J, et al. Study protocol for a randomised controlled trial evaluating the effect of prenatal omega‐3 LCPUFA supplementation to reduce the incidence of preterm birth: the ORIP trial. BMJ Open 2017;7(9):e018360. [doi: 10.1136/bmjopen‐2017‐018360] - PMC - PubMed
Martini 2014 (CORDHA) {published data only}
-
- ISRCTN58396079. A randomised controlled trial for the optimization of the viability of stem cells derived from umbilical cord blood after maternal supplementation with DHA during the second or third trimester of pregnancy. isrctn.com/ISRCTN58396079 (first received 8 October 2013). [ISRCTN58396079] - PMC - PubMed
-
- Martini I, Domenico EG, Scala R, Caruso F, Ferreri C, Ubaldi FM, et al. Optimization of the viability of stem cells derived from umbilical cord blood after maternal supplementation with DHA during the second or third trimester of pregnancy: study protocol for a randomized controlled trial. Trials 2014;15:164. [ISRCTN58396079] - PMC - PubMed
Mbayiwa 2016 {published data only}
-
- Mbayiwa K, NCT02647723. Improving maternal and child health through prenatal fatty acid supplementation: a randomized controlled study in African American women living in low‐income urban environments. clinicaltrials.gov/show/NCT02647723 (first received 6 January 2016). [NCT02647723]
Murff 2017 (FORTUNE) {published data only}
-
- Murff HJ. Fish oil to reduce tobacco use in expectant mothers study. clinicaltrials.gov/ct2/show/record/NCT03077724 (first received 13 March 2017). [NCT03077724]
Nishi 2015 (SYNCHRO) {unpublished data only}
-
- Nishi D, NCT02166424. The synchronized trial on expectant mothers with depressive symptoms by omega‐3 PUFAs (SYNCHRO). clinicaltrials.gov/show/NCT02166424 (first received 15 June 2014). [NCT02166424]
Wang 2018 {published data only}
-
- Wang WJ, NCT03569501. Influence of DHA/oat on maternal and neonatal metabolic health in gestational diabetes mellitus. clinicaltrials.gov/ct2/show/NCT03569501 (first received 26 June 2018).
Zielinsky 2015 {published data only}
-
- Zielinsky P, NCT02565290. Effect of mother's supplementation omega‐3 in the dynamics of fetal ductus arteriosus: a randomized clinical trial. clinicaltrials.gov/ct2/show/NCT02565290 (first received 30 September 2015). [NCT02565290]
Zimmermann 2018 {published data only}
-
- Zimmermann JB, RBR‐5kbzdh. Dietary supplementation of omega 3 and the participation in the placentary vascular resistance mechanism in pregnant people: a comparison with normal pregnant women without use of omega, chronic hypertensions in use of AAS and pregnants with thrombophilia and use heparina or AAS. ensaiosclinicos.gov.br/rg/RBR‐5kbzdh/ (first received 13 June 2018).
Additional references
Borge 2017
Brantsaeter 2017
Campoy 2012
Chen 2015
-
- Chen B, Ji X, Zhang L, Hou Z, Li C, Tong Y. Fish oil supplementation does not reduce risks of gestational diabetes mellitus, pregnancy‐induced hypertension, or pre‐eclampsia: a meta‐analysis of randomized controlled trials. Medical Science Monitor 2015;21:2322‐30. [DOI: 10.12659/MSM.894033] - DOI - PMC - PubMed
Chen 2016
Conroy 2012
-
- Conroy S, Pariante CM, Marks MN, Davies HA, Farrelly S, Schacht R, et al. Maternal psychopathology and infant development at 18 months: the impact of maternal personality disorder and depression. Journal of the American Academy of Child Adolescent Psychiatry 2012;51(1):51‐61. - PubMed
Dennis 2012
-
- Dennis CL, Heaman M, Vigod S. Epidemiology of postpartum depressive symptoms among Canadian women: regional and national results from a cross‐sectional survey. Canadian Journal of Psychiatry 2012;57(9):537‐46. - PubMed
Gaynes 2005
-
- Gavin NI, Gaynes BN, Lohr KN, Meltzer‐Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstetrics and Gynecology 2005;106(5 Pt 1):1071‐83. - PubMed
Golding 2009
Gould 2013
-
- Gould JF, Smithers LG, Makrides M. The effect of maternal omega‐3 (n‐3) LCPUFA supplementation during pregnancy on early childhood cognitive and visual development: a systematic review and meta‐analysis of randomized controlled trials. American Journal of Nutrition 2013;97(3):531‐44. [DOI: 10.3945/ajcn.112.045781] - DOI - PubMed
Greenberg 2008
Gunaratne 2015
Hibbeln 2007
-
- Hibbeln JR, Davis JM, Steer C, Emmett P, Rogers I, Williams C, et al. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study. Lancet 2007;369:578‐85. - PubMed
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Imhoff‐Kunsch 2012
-
- Imhoff‐Kunsch B, Briggs V, Goldenberg T, Ramakrishnan U. Effect of n‐3 long‐chain polyunsaturated fatty acid intake during pregnancy on maternal, infant, and child health outcomes: a systematic review. Paediatric and Perinatal Epidemiology 2012;26(Suppl 1):91‐107. [doi: 10.1111/j.1365‐3016.2012.01292.x] - PubMed
Jensen 2006
-
- Jensen CL. Effects of n‐3 fatty acids during pregnancy and lactation. American Journal of Clinical Nutrition 2006;83:1452S‐7S. - PubMed
Kar 2016
-
- Kar S, Wong M, Rogozinska E, Thangaratinam S. Effects of omega‐3 fatty acids in prevention of early preterm delivery: a systematic review and meta‐analysis of randomized studies. European Journal of Obstetrics, Gynecology and Reproductive Biology 2016;198:40‐6. [DOI: 10.1016/j.ejogrb.2015.11.033] - DOI - PubMed
Koletzko 2007
-
- Koletzko B, Cetin I, Brenna JT, the Perinatal Lipid Intake Working Group. Consensus statement: dietary fat intakes for pregnant and lactating women. British Journal of Nutrition 2007;98:873‐7. - PubMed
Leghi 2016
-
- Leghi GE, Muhlhausler BS. The effect of n‐3 LCPUFA supplementation on oxidative stress and inflammation in the placenta and maternal plasma during pregnancy. Prostaglandins, Leukotrienes and Essential Fatty Acids 2016;113:33‐9. - PubMed
Leventakou 2014
Li 2017
Liu 2016
-
- Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, et al. Global, regional, and national causes of under‐5 mortality in 2000‐15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet 2016;388(10063):3027‐35. [DOI: 10.1016/S0140-6736(16)31593-8] - DOI - PMC - PubMed
Meyer 2016
Mol 2016
Newberry 2016
-
- Newberry SJ, Chung M, Booth M, Maglione M, Tang AM, O'Hanlon CE, et al. Omega‐3 fatty acids and maternal and child health: an updated systematic review. AHRQ Publication No. 16‐E003‐EF. Vol. Evidence Report/Technology Assessment No. 224, Rockville (MD): Agency for Healthcare Research and Quality, 2016. [DOI: ] - PubMed
Nordgren 2017
Oken 2018
-
- Oken E. Fish consumption and docosahexaenoic acid (DHA) supplementation in pregnancy. UpToDate (www.uptodate.com) 2018.
Olsen 1985
Olsen 1986
-
- Olsen SF, Hansen HS, Sørensen TI, Jensen B, Secher NJ, Sommer S, et al. Intake of marine fat, rich in (n‐3)‐polyunsaturated fatty acids, may increase birthweight by prolonging gestation. Lancet 1986;2(8503):367‐9. - PubMed
Olsen 1991
-
- Olsen SF, Hansen HS, Sommer S, Jensen B, Sørensen TI, Secher NJ, et al. Gestational age in relation to marine n‐3 fatty acids in maternal erythrocytes: a study of women in the Faroe Islands and Denmark. American Journal of Obstetrics and Gynecology 1991;164(5 Pt 1):1203‐9. - PubMed
Olsen 2002
Olsen 2006
-
- Olsen SF, Østerdal ML, Salvig JD, Kesmodel U, Henriksen TB, Hedegaard M, et al. Duration of pregnancy in relation to seafood intake during early and mid pregnancy: prospective cohort. European Journal of Epidemiology 2006;21(10):749‐58. - PubMed
Palmer 2012
Rangel‐Huerta 2018
Review Manager 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Saccone 2015a
-
- Saccone G, Berghella V, Maruotti GM, Sarno L, Martinelli P. Omega‐3 supplementation during pregnancy to prevent recurrent intrauterine growth restriction: systematic review and meta‐analysis of randomized controlled trials. Ultrasound Obstetrics and Gynecology 2015;46(6):659‐64. [DOI: 10.1002/uog.14910] - DOI - PubMed
Saccone 2015b
-
- Saccone G, Berghella V. Omega‐3 supplementation to prevent recurrent preterm birth: a systematic review and metaanalysis of randomized controlled trials. American Journal of Obstetrics and Gynecology 2015;213(2):135‐40. - PubMed
Saccone 2015c
Saccone 2016
Saigal 2008
-
- Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2008;371(9608):261‐9. - PubMed
Shulkin 2018
-
- Shulkin M, Pimpin L, Bellinger D, Kranz S, Fawzi W, Duggan C, et al. n‐3 fatty acid supplementation in mothers, preterm Infants, and term Infants and childhood psychomotor and visual development: a systematic review and meta‐analysis. Journal of Nutrition 2018;148(3):409‐18. [DOI: 10.1093/jn/nxx031] - DOI - PMC - PubMed
Stillbirth CRE 2018
-
- Stillbirth CR. Position statement: detection and management of fetal growth restriction in singleton pregnancies. Stillbirth CR 2018.
Thornton 2008
-
- Thornton S. Preterm Birth: Causes, Consequences and Prevention; The Obstetrician & Gynaecologist. Wiley, 2008. [DOI: 10.1576/toag.10.4.280b.27453] - DOI
Vaz Jdos 2013
-
- Vaz Jdos S, Kac G, Emmett P, Davis JM, Golding J, Hibbeln JR. Dietary patterns, n‐3 fatty acids intake from seafood and high levels of anxiety symptoms during pregnancy: findings from the Avon Longitudinal Study of Parents and Children. PLOS One 2013;8(7):e67671. [doi: 10.1371/journal.pone.0067671] - PMC - PubMed
Westrupp 2014
-
- Westrupp EM, Lucas N, Mensah FK, Gold L, Wake M, Nicholson JM. Community‐based healthcare costs for children born low birthweight, preterm and/or small for gestational age: data from the Longitudinal Study of Australian Children. Child Care Health Development 2014;40(2):259‐66. [DOI: 10.1111/cch.12040] - DOI - PubMed
World Health Organization 2017
-
- World Health Organization. WHO fact sheet: Preterm birth. www.who.int/mediacentre/factsheets/fs363/en/ (accessed 15 April 2018).
Zhou 2017
-
- Zhou SJ, Best K, Gibson R, McPhee A, Yelland L, Quinlivan J, et al. Study protocol for a randomised controlled trial evaluating the effect of prenatal omega‐3 LCPUFA supplementation to reduce the incidence of preterm birth: the ORIP trial. BMJ Open 2017;7:e018360. [doi: 10.1136/bmjopen‐2017‐018360] - PMC - PubMed
Zhu 2014
-
- Zhu P, Sun MS, Hao JH, Chen YJ, Jiang XM, Tao RX, et al. Does prenatal maternal stress impair cognitive development and alter temperament characteristics in toddlers with healthy birth outcomes?. Developmental Medicine & Child Neurology 2014;56(3):283‐9. - PubMed
References to other published versions of this review
Duley 1995
-
- Duley L. Prophylactic fish oil in pregnancy. [revised 21 April 1994] In: Enkin MW, Keirse MJ, Renfrew MJ, Neilson JP, Crowther CA, editor(s) Pregnancy and Childbirth Module. The Cochrane Pregnancy and Childbirth Database [database on disk and CDROM]. The Cochrane Collaboration, Issue 2, 1995. Oxford: Update Software.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

