Probiotics for treating eczema
- PMID: 30480774
- PMCID: PMC6517242
- DOI: 10.1002/14651858.CD006135.pub3
Probiotics for treating eczema
Abstract
Background: Eczema is a common chronic skin condition. Probiotics have been proposed as an effective treatment for eczema; their use is increasing, as numerous clinical trials are under way. This is an update of a Cochrane Review first published in 2008, which suggested that probiotics may not be an effective treatment for eczema but identified areas in which evidence was lacking.
Objectives: To assess the effects of probiotics for treating patients of all ages with eczema.
Search methods: We updated our searches of the following databases to January 2017: the Cochrane Skin Group Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), in the Cochrane Library, the Global Resource of Eczema Trials (GREAT) database, MEDLINE, Embase, PsycINFO, the Allied and Complementary Medicine Database (AMED), and Latin American Caribbean Health Sciences Literature (LILACS). We searched five trials registers and checked the reference lists of included studies and relevant reviews for further references to relevant randomised controlled trials (RCTs). We also handsearched a number of conference proceedings. We updated the searches of the main databases in January 2018 and of trials registries in March 2018, but we have not yet incorporated these results into the review.
Selection criteria: Randomised controlled trials of probiotics (live orally ingested micro-organisms) compared with no treatment, placebo, or other active intervention with no probiotics for the treatment of eczema diagnosed by a doctor.
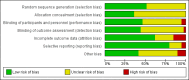
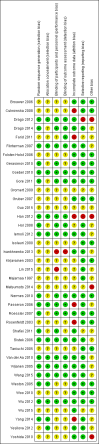
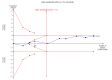
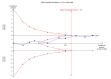
Data collection and analysis: We used standard methodological procedures as expected by Cochrane. We recorded adverse events from the included studies and from a separate adverse events search conducted for the first review. We formally assessed reporting bias by preparing funnel plots, and we performed trial sequential analysis for the first primary outcome - eczema symptoms at the end of active treatment.We used GRADE to assess the quality of the evidence for each outcome (in italic font).
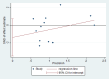
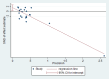
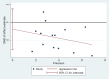
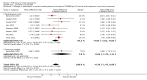
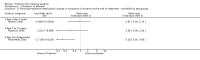
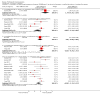
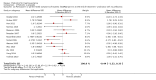
Main results: We included 39 randomised controlled trials involving 2599 randomised participants. We included participants of either gender, aged from the first year of life through to 55 years (only six studies assessed adults), who had mild to severe eczema. Trials were undertaken in primary and secondary healthcare settings, mainly in Europe or Asia. Duration of treatment ranged from four weeks to six months, and duration of follow-up after end of treatment ranged from zero to 36 months. We selected no standard dose: researchers used a variety of doses and concentrations of probiotics. The probiotics used were bacteria of the Lactobacillus and Bifidobacteria species, which were taken alone or combined with other probiotics, and were given with or without prebiotics. Comparators were no treatment, placebo, and other treatments with no probiotics.For all results described in this abstract, the comparator was no probiotics. Active treatment ranged from six weeks to three months for all of the following results, apart from the investigator-rated eczema severity outcome, for which the upper limit of active treatment was 16 weeks. With regard to score, the higher the score, the more severe were the symptoms. All key results reported in this abstract were measured at the end of active treatment, except for adverse events, which were measured during the active treatment period.Probiotics probably make little or no difference in participant- or parent-rated symptoms of eczema (13 trials; 754 participants): symptom severity on a scale from 0 to 20 was 0.44 points lower after probiotic treatment (95% confidence interval (CI) -1.22 to 0.33; moderate-quality evidence). Trial sequential analysis shows that target sample sizes of 258 and 456, which are necessary to demonstrate a minimum mean difference of -2 and -1.5, respectively, with 90% power, have been exceeded, suggesting that further trials with similar probiotic strains for this outcome at the end of active treatment may be futile.We found no evidence suggesting that probiotics make a difference in QoL for patients with eczema (six studies; 552 participants; standardised mean difference (SMD) 0.03, 95% CI -0.36 to 0.42; low-quality evidence) when measured by the participant or the parent using validated disease-specific QoL instruments.Probiotics may slightly reduce investigator-rated eczema severity scores (24 trials; 1596 participants). On a scale of 0 to 103 for total Severity Scoring of Atopic Dermatitis (SCORAD), a score combining investigator-rated eczema severity score and participant scoring for eczema symptoms of itch and sleep loss was 3.91 points lower after probiotic treatment than after no probiotic treatment (95% CI -5.86 to -1.96; low-quality evidence). The minimum clinically important difference for SCORAD has been estimated to be 8.7 points.We noted significant to extreme levels of unexplainable heterogeneity between the results of individual studies. We judged most studies to be at unclear risk of bias; six studies had high attrition bias, and nine were at low risk of bias overall.We found no evidence to show that probiotics make a difference in the risk of adverse events during active treatment (risk ratio (RR) 1.54, 95% CI 0.90 to 2.63; seven trials; 402 participants; low-quality evidence). Studies in our review that reported adverse effects described gastrointestinal symptoms.
Authors' conclusions: Evidence suggests that, compared with no probiotic, currently available probiotic strains probably make little or no difference in improving patient-rated eczema symptoms. Probiotics may make little or no difference in QoL for people with eczema nor in investigator-rated eczema severity score (combined with participant scoring for eczema symptoms of itch and sleep loss); for the latter, the observed effect was small and of uncertain clinical significance. Therefore, use of probiotics for the treatment of eczema is currently not evidence-based. This update found no evidence of increased adverse effects with probiotic use during studies, but a separate adverse events search from the first review revealed that probiotic treatment carries a small risk of adverse events.Results show significant, unexplainable heterogeneity between individual trial results. Only a small number of studies measured some outcomes.Future studies should better measure QoL scores and adverse events, and should report on new probiotics. Researchers should also consider studying subgroups of patients (e.g. patients with atopy or food allergies, adults) and standardising doses/concentrations of probiotics given.
Conflict of interest statement
Robert Boyle: "I have received speaker honoraria, support for travel to a conference, and a research grant from Danone Research, whose parent company market products containing probiotics. I undertook a consultancy project for an infant formula company (Dairy Goat Cooperative) in 2017, to help them design a robust and ethical clinical trial of an infant formula milk. The project related to prevention of eczema, but did not involve probiotics or eczema treatment". Mimi Tang: "My conflicts of interest are as follows:
past member of global scientific advisory board Danone Nutricia (resigned December 2016);
past member of medical advisory board for Oceania Nestle Nutrition Institute (resigned);
speaker fees at symposia sponsored by Danone Nutricia, Abbott, and Nestle Health Science;
consultant to Deerfield, GLG, and Bayer;
employee with shares/share options Prota Therapeutics;
inventor on a patent owned by MCRI (Murdoch Children's Research Institute);
grant, received from my institution, from Prota Therapeutics;
royalties from Wiley, as an author of a book
Kids Food Allergies for Dummies ; andpayment for development of educational presentations from MD Linx ‐ I developed a GP education module: 'Microbiota and Immune Development'".
Areti Makrygeorgou: "I have received speaker honorarium by Celgene and support to travel to a conference by Novartis". Jo Leonardi‐Bee: nothing to declare. Fiona J Bath‐Hextall: nothing to declare. Dedee F Murrell: "I run a clinical trials centre for a variety of skin diseases, including atopic dermatitis and give lectures on this topic. I am a Councillor (a voluntary position) representing Australia on the International Eczema Council. I have received travel expenses and payment for lectures from Sanofi. My institution has received grants for my role as investigator on atopic dermatitis clinical trials assessing crisaborole (Anacor Pharmaceuticals), dupilumab (Regeneron), tralokinumab (MedImmune), and nemolizumab (Galderma)." Amanda Roberts: nothing to declare. Nerys Roberts (clinical referee): "I am the Steering Committee chair of the Atopic Dermatitis Anti‐IgE Paediatric Trial (ADAPT)". Raja Sivamani (clinical referee): "I serve as a Scientific Advisor for Dermveda".
Figures
























Update of
-
Probiotics for treating eczema.Cochrane Database Syst Rev. 2008 Oct 8;(4):CD006135. doi: 10.1002/14651858.CD006135.pub2. Cochrane Database Syst Rev. 2008. Update in: Cochrane Database Syst Rev. 2018 Nov 21;11:CD006135. doi: 10.1002/14651858.CD006135.pub3. PMID: 18843705 Updated.
Comment in
-
Cochrane Corner: Probiotics: make little or no difference in patient rated symptoms for eczema.J Prim Health Care. 2018 Dec;10(4):352-353. doi: 10.1071/HC15938. J Prim Health Care. 2018. PMID: 31039966 No abstract available.
-
From the Cochrane Library: Probiotics for treating eczema.J Am Acad Dermatol. 2022 Mar;86(3):e127-e132. doi: 10.1016/j.jaad.2021.10.032. Epub 2021 Nov 5. J Am Acad Dermatol. 2022. PMID: 34748863 No abstract available.
References
References to studies included in this review
Brouwer 2006 {published and unpublished data}
-
- Brouwer ML, Wolt‐Plompen SA, Dubois AE, Heide S, Jansen DF, Hoijer MA, et al. No effects of probiotics on atopic dermatitis in infancy: a randomized placebo‐controlled trial. Clinical and Experimental Allergy 2006;36(7):899‐906. [CENTRAL: CN‐00571157; PUBMED: 16839405] - PubMed
Cukrowska 2008 {published data only}
-
- Cukrowska B, Ceregra A, Klewicka E, Slizewska K, Motyl I, Libudzisz Z. Probiotic lactobacillus casei and lactobacillus paracasei strains in treatment of food allergy in children [Probiotyczne szczepy lactobacillus casei i lactobacillus paracasei w leczeniu alergii pokarmowej u dzieci]. Przegląd Pediatryczny 2010;40(1):21‐5. [CENTRAL: CN‐00803280]
-
- Cukrowska B, Ceregra A, Rosiak I, Klewicka E, Slizewska K, Motyl I, et al. The influence of probiotic Lactobacillus casei and paracasei strains on clinical status of atopic eczema in children with food allergy on cow's milk proteins [Wpływ probiotycznych szczepów Lactobacillus casei i paracasei na przebieg kliniczny wyprysku atopowego u dzieci z alergią pokarmową na białka mleka krowiego]. Pediatria Wspolczesna 2008;10(2):67‐70. [CENTRAL: CN‐00707920]
Drago 2012 {published and unpublished data}
-
- Drago L, Iemoli E, Rodighiero V, Nicola L, Vecchi E, Piconi S. Effects of Lactobacillus salivarius LS01 (DSM 22775) treatment on adult atopic dermatitis: a randomized placebo‐controlled study. International Journal of Immunopathology and Pharmacology 2011;24(4):1037‐48. [CENTRAL: CN‐00843712; PUBMED: 22230409] - PubMed
-
- Drago L, Toscano M, Vecchi E, Piconi S, Iemoli E. Changing of fecal flora and clinical effect of L. salivarius LS01 in adults with atopic dermatitis. Journal of Clinical Gastroenterology 2012;46(Suppl):S56‐S63. [CENTRAL: CN‐00967749; PUBMED: 22955359] - PubMed
Drago 2014 {published data only}
-
- Drago L, Vecchi E, Toscano M, Vassena C, Altomare G, Pigatto P. Treatment of atopic dermatitis eczema with a high concentration of Lactobacillus salivarius LS01 associated with an innovative gelling complex: a pilot study on adults. Journal of Clinical Gastroenterology 2014;48(Suppl 1):S47‐51. [CENTRAL: CN‐01113253] - PubMed
Farid 2011 {published data only}
Flinterman 2007 {published and unpublished data}
-
- Flinterman AE, Knol EF, Ieperen‐van Dijk AG, Timmerman HM, Knulst AC, Bruijnzeel‐Koomen CA, et al. Probiotics have a different immunomodulatory potential in vitro versus ex vivo upon oral administration in children with food allergy. International Archives of Allergy and Immunology 2007;143(3):237‐44. [PUBMED: 17290150] - PubMed
Folster‐Holst 2006 {published data only}
-
- Folster‐Holst R, Muller F, Schnopp N, Abeck D, Kreiselmaier I, Lenz T, et al. Prospective, randomized controlled trial on Lactobacillus rhamnosus in infants with moderate to severe atopic dermatitis. British Journal of Dermatology 2006;155(6):1256‐61. [CENTRAL: CN‐00576925; PUBMED: 17107398] - PubMed
Gerasimov 2010 {published data only}
-
- Gerasimov SV, Vasjuta VV, Myhovych OO, Bondarchuk LI. Probiotic supplement reduces atopic dermatitis in preschool children: a randomized, double‐blind, placebo‐controlled, clinical trial. American Journal of Clinical Dermatology 2010;11(5):351‐61. [CENTRAL: CN‐00762545; PUBMED: 20642296] - PubMed
Goebel 2010 {published and unpublished data}
-
- Gobel R, Larsen, N, Molgaard C, Jakobsen M, Michaelsen KF. Probiotics to young children with atopic dermatitis: a randomized placebo‐controlled trial. International Journal of Probiotics and Prebiotics 2010;5(2):53‐60. [CENTRAL: CN‐00803376]
-
- Larsen N, Vogensen FK, Gøbel R, Michaelsen KF, Abu Al‐Soud W, Sørensen SJ, et al. Predominant genera of fecal microbiota in children with atopic dermatitis are not altered by intake of probiotic bacteria Lactobacillus acidophilus NCFM and Bifidobacterium animalis subsp lactis Bi‐07. FEMS Microbiology Ecology 2011;75(3):482‐96. [CENTRAL: CN‐00771294; PUBMED: 21204871] - PubMed
Gore 2011 {published and unpublished data}
-
- Gore C, Custovic A, Tannock GW, Munro K, Kerry G, Johnson K, et al. Treatment and secondary prevention effects of the probiotics Lactobacillus paracasei or Bifidobacterium lactis on early infant eczema: randomized controlled trial with follow‐up until age 3 years. Clinical and Experimental Allergy 2011;42(1):112‐22. [CENTRAL: CN‐00843786; PUBMED: 22092692] - PubMed
Gromert 2009 {published data only}
-
- Gromert N, Axelsson I. Lactobacillus reuteri effect on atopic eczema in childhood. Journal of Pediatric Gastroenterology and Nutrition 2009;43(Suppl 3):E148‐9. [CENTRAL: CN‐00980761]
Gruber 2007 {published data only (unpublished sought but not used)}
-
- Gruber C, Wendt M, Sulser C, Lau S, Kulig M, Wahn U, et al. Randomised, placebo‐controlled trial of Lactobacillus rhamnosus GG as treatment of atopic dermatitis in infancy. Allergy 2007;62(11):1270‐6. [CENTRAL: CN‐00619933; PUBMED: 17919141] - PubMed
Guo 2015 {published data only}
-
- Guo Y‐H, Mou Y‐D, Wang H‐S, Guo Y‐J. Clinical effect of microecologics as an adjuvant therapy on infants' eczema. Journal of Dalian Medical University 2015;37(6):571‐88. [DOI: 10.11724/jdmu.2015.06.13] - DOI
Han 2012 {published and unpublished data}
-
- Han Y, Kim B, Ban J, Lee J, Kim BJ, Choi BS, et al. A randomized trial of Lactobacillus plantarum CJLP133 for the treatment of atopic dermatitis. Pediatric Allergy and Immunology 2012;23(7):667‐73. [CENTRAL: CN‐00871398; PUBMED: 23050557] - PubMed
Hol 2008 {published data only}
-
- Hol J, Leer EH, Elink Schuurman BE, Ruiter LF, Samsom JN, Hop W, et al. The acquisition of tolerance toward cow's milk through probiotic supplementation: a randomized, controlled trial. Journal of Allergy and Clinical Immunology 2008;121(6):1448‐54. [CENTRAL: CN‐00639187; PUBMED: 18436293] - PubMed
Iemoli 2012 {published and unpublished data}
-
- Iemoli E, Trabattoni D, Parisotto S, Borgonovo L, Toscano M, Rizzardini G, et al. Probiotics reduce gut microbial translocation and improve adult atopic dermatitis. Journal of Clinical Gastroenterology 2012;46(Suppl):S33‐40. [CENTRAL: CN‐00871399; PUBMED: 22955355] - PubMed
Isolauri 2000 {published data only}
-
- Isolauri E, Arvola T, Sutas Y, Moilanen E, Salminen S. Probiotics in the management of atopic eczema. Clinical and Experimental Allergy 2000;30(11):1604‐10. [CENTRAL: CN‐00330163; PUBMED: 11069570] - PubMed
Ivankhnenko 2013 {published data only}
-
- Ivakhnenko O, Niankovskyy S. Clinical effectiveness of probiotics in complex treatment of infants with cow's milk allergy. Georgian Medical News 2013;216:39‐45. [CENTRAL: CN‐01123797; PUBMED: 23567307] - PubMed
Kirjavainen 2003 {published data only}
-
- Kirjavainen PV, Salminen SJ, Isolauri E. Probiotic bacteria in the management of atopic disease: underscoring the importance of viability. Journal of Pediatric Gastroenterology and Nutrition 2003;36(2):223‐7. [CENTRAL: CN‐00435079; PUBMED: 12548058] - PubMed
Lin 2015 {published data only}
Majamaa 1997 {published data only}
-
- Majamaa H, Isolauri E. Probiotics: a novel approach in the management of food allergy. Journal of Allergy and Clinical Immunology 1997;99(2):179‐85. [CENTRAL: CN‐00136911; PUBMED: 9042042] - PubMed
Matsumoto 2014 {published data only}
-
- Matsumoto M, Ebata T, Hirooka J, Hosoya R, Inoue N, Itami S, et al. Antipruritic effects of the probiotic strain LKM512 in adults with atopic dermatitis. Annals of Allergy, Asthma and Immunology 2014;113(2):209‐16. [CENTRAL: CN‐00998958; PUBMED: 24893766] - PubMed
Nermes 2010 {published and unpublished data}
-
- Nermes M, Kantele JM, Atosuo TJ, Salminen S, Isolauri E. Interaction of orally administered Lactobacillus rhamnosus GG with skin and gut microbiota and humoral immunity in infants with atopic dermatitis. Clinical and Experimental Allergy 2010;41(3):370‐7. [CENTRAL: CN‐00779496; PUBMED: 21121981] - PubMed
Passeron 2006 {published and unpublished data}
-
- Passeron T, Lacour J‐P, Fontas E, Ortonne JP. Prebiotics and synbiotics: two promising approaches for the treatment of atopic dermatitis in children above 2 years. Allergy 2006;61(4):431‐7. [CENTRAL: CN‐00563037; PUBMED: 16512804] - PubMed
Roessler 2007 {published and unpublished data}
-
- Roessler A, Forssten SD, Glei M, Ouwehand AC, Jahreis G. The effect of probiotics on faecal microbiota and genotoxic activity of faecal water in patients with atopic dermatitis: a randomized, placebo‐controlled study. Clinical Nutrition 2012;31(1):22‐9. [CENTRAL: CN‐00882332; PUBMED: 21963389] - PubMed
-
- Roessler A, Friedrich U, Vogelsang H, Bauer A, Kaatz M, Hipler UC, et al. The immune system in healthy adults and patients with atopic dermatitis seems to be affected differently by a probiotic intervention. Clinical and Experimental Allergy 2008;38(1):93‐102. [CENTRAL: CN‐00621150; PUBMED: 18028460] - PubMed
Rosenfeldt 2003 {published and unpublished data}
-
- Rosenfeldt V, Benfeldt E, Nielsen SD, Michaelsen KF, Jeppesen DL, Valerius NH, et al. Effect of probiotic Lactobacillus strains in children with atopic dermatitis. Journal of Allergy and Clinical Immunology 2003;111(2):389‐95. [CENTRAL: CN‐00413280; PUBMED: 12589361] - PubMed
-
- Rosenfeldt V, Benfeldt E, Valerius NH, Paeregaard A, Michaelsen KF. Effects of probiotics on gastrointestinal symptoms and small intestinal permeability in children with atopic dermatitis. Journal of Pediatrics 2004;145(5):612‐6. [CENTRAL: CN‐00492535; CRSREF: 3073882; PUBMED: 15520759] - PubMed
Shafiei 2011 {published data only}
-
- Shafiei A, Moin M, Pourpak Z, Gharagozlou M, Aghamohammadi A, Aghamohamadi A, et al. Synbiotics could not reduce the scoring of childhood atopic dermatitis (SCORAD): a randomized double blind placebo‐controlled trial. Iranian Journal of Allergy, Asthma and Immunology 2011;10(1):21‐8. [CENTRAL: CN‐00801473; PUBMED: 21358011] - PubMed
Sistek 2006 {published and unpublished data}
-
- Sistek D, Kelly R, Wickens K, Stanley T, Fitzharris P, Crane J. Is the effect of probiotics on atopic dermatitis confined to food sensitized children?. Clinical and Experimental Allergy 2006;36(5):629‐33. [CENTRAL: CN‐00564896; PUBMED: 16650048] - PubMed
Taniuchi 2005 {published data only}
-
- Hattori K, Yamamoto A, Sasai M, Taniuchi S, Kojima T, Kobayashi Y, et al. Effects of administration of bifidobacteria on fecal microflora and clinical symptoms in infants with atopic dermatitis. Arerugi [Allergy] 2003;52(1):20‐30. [CENTRAL: CN‐00558320; PUBMED: 12598719] - PubMed
-
- Taniuchi S, Hattori K, Yamamoto A, Sasai M, Hatano Y, Kojima T, et al. Administration of Bifidobacterium to infants with atopic dermatitis: changes in fecal microflora and clinical symptoms. Journal of Applied Research 2005;5(2):387‐96. [CENTRAL: CN‐00569597]
Van der Aa 2010 {published and unpublished data}
-
- Aa LB, Aalderen WM, Heymans HS, Henk Sillevis Smitt J, Nauta AJ, Knippels LM, et al. Synbiotics prevent asthma‐like symptoms in infants with atopic dermatitis. Allergy 2011;66(2):170‐7. [CENTRAL: CN‐00779370; PUBMED: 20560907] - PubMed
-
- Aa LB, Heymans HS, Aalderen WM, Sillevis Smitt JH, Knol J, Ben Amor K, et al. Effect of a new synbiotic mixture on atopic dermatitis in infants: a randomized‐controlled trial. Clinical and Experimental Allergy 2010;40(5):795‐804. [CENTRAL: CN‐00761390; PUBMED: 20184604] - PubMed
-
- Aa LB, Lutter R, Heymans HS, Smids BS, Dekker T, Aalderen WM, et al. No detectable beneficial systemic immunomodulatory effects of a specific synbiotic mixture in infants with atopic dermatitis. Clinical and Experimental Allergy 2012;42(4):531‐9. [CENTRAL: CN‐00839910; PUBMED: 22092915] - PubMed
Viljanen 2005 {published and unpublished data}
-
- Pohjavuori E, Viljanen M, Korpela R, Kuitunen M, Tiittanen M, Vaarala O, et al. Lactobacillus GG effect in increasing IFN‐gamma production in infants with cow's milk allergy. Journal of Allergy and Clinical Immunology 2004;114(1):131‐6. [CENTRAL: CN‐00480315; PUBMED: 15241356] - PubMed
-
- Viljanen M, Kuitunen M, Haahtela T, Juntunen‐Backman K, Korpela R, Savilahti E. Probiotic effects on faecal inflammatory markers and on faecal IgA in food allergic atopic eczema/dermatitis syndrome infants. Pediatric Allergy and Immunology 2005;16(1):65‐71. [CENTRAL: CN‐00520986; PUBMED: 15693914] - PubMed
-
- Viljanen M, Pohjavuori E, Haahtela T, Korpela R, Kuitunen M, Sarnesto A, et al. Induction of inflammation as a possible mechanism of probiotic effect in atopic eczema‐dermatitis syndrome. Journal of Allergy & Clinical Immunology 2005;115(6):1254‐9. [CENTRAL: CN‐00515574; PUBMED: 15940143] - PubMed
-
- Viljanen M, Savilahti E, Haahtela T, Juntunen‐Backman K, Korpela R, Poussa T, et al. Probiotics in the treatment of atopic eczema/dermatitis syndrome in infants: a double‐blind placebo‐controlled trial. Allergy 2005;60(4):494‐500. [CENTRAL: CN‐00513132; PUBMED: 15727582] - PubMed
Wang 2015 {published data only}
-
- Wang IJ, Wang JY. Children with atopic dermatitis show clinical improvement after Lactobacillus exposure. Clinical & Experimental Allergy 2015;45(4):779‐87. [CENTRAL: CN‐01069031; PUBMED: 25600169] - PubMed
Weston 2005 {published and unpublished data}
-
- Prescott SL, Dunstan JA, Hale J, Breckler L, Lehmann H, Weston S, et al. Clinical effects of probiotics are associated with increased interferon‐gamma responses in very young children with atopic dermatitis. Clinical and Experimental Allergy 2005;35(12):1557‐64. [PUBMED: 16393321] - PubMed
-
- Weston S, Dunstan J, Roper J, Breckler L, Halbert A, Richmond P, et al. Probiotics provide clinical benefit in moderate and severe atopic dermatitis: a randomised controlled trial. Journal of Investigative Dermatology 2005;125:596.
-
- Weston S, Richmond P, Halbert A, Prescott SL. Effects of probiotics in infants with atopic dermatitis: a randomised double blind placebo controlled trial. Australasian Journal of Dermatology 2004;45:A13. [CENTRAL: CN‐00594250]
Woo 2010 {published data only}
-
- Woo SI, Kim JY, Lee YJ, Kim NS, Hahn YS. Effect of Lactobacillus sakei supplementation in children with atopic eczema‐dermatitis syndrome. Annals of Allergy, Asthma and Immunology 2010;104(4):343‐8. [CENTRAL: CN‐00742516; PUBMED: 20408346] - PubMed
Wu 2012 {published data only}
-
- Wu KG, Li TH, Peng HJ. Lactobacillus salivarius plus fructo‐oligosaccharide is superior to fructo‐oligosaccharide alone for treating children with moderate to severe atopic dermatitis: a double‐blind, randomized, clinical trial of efficacy and safety. British Journal of Dermatology 2012;166(1):129‐36. [CENTRAL: CN‐00841293; PUBMED: 21895621] - PubMed
Wu 2015 {published data only}
-
- Wu Y‐J, Wu W‐F, Hung C‐W, Ku M‐S, Liao P‐F, Sun H‐L, et al. Evaluation of efficacy and safety of Lactobacillus rhamnosus in children aged 4‐48 months with atopic dermatitis: an 8‐week, double‐blind, randomized, placebo‐controlled study. Journal of Microbiology, Immunology and Infection 2017;50(5):684‐92. [DOI: 10.1016/j.jmii.2015.10.003; PUBMED: 26733351] - DOI - PubMed
Yang 2014 {published data only}
Yesilova 2012 {published data only}
Yoshida 2010 {published data only}
-
- Yoshida Y, Seki T, Matsunaka H, Watanabe T, Shindo M, Yamada N, et al. Clinical effects of probiotic Bifidobacterium breve supplementation in adult patients with atopic dermatitis. Yonago Acta Medica 2010;53(2):37‐45. [CENTRAL: CN‐00865095]
References to studies excluded from this review
Arkwright 2003 {published data only}
-
- Arkwright PD, David TJ. Effect of Mycobacterium vaccae on atopic dermatitis in children of different ages. British Journal of Dermatology 2003;149(5):1029‐34. [CENTRAL: CN‐00472817; PUBMED: 14632810] - PubMed
Arvola 2006 {published data only}
-
- Arvola T, Ruuska T, Keranen J, Hyoty H, Salminen S, Isolauri E. Rectal bleeding in infancy: clinical, allergological, and microbiological examination. Pediatrics 2006;117(4):e760‐8. [CENTRAL: CN‐00556020; PUBMED: 16585287] - PubMed
Aryayev 2006 {published and unpublished data}
-
- Aryayev ML, Kukushkin NV. Combined therapy with pimecrolimus cream 1% and probiotic in children with atopic dermatitis. European Journal of Pediatrics 2006;165(Suppl 1):53. [CENTRAL: CN‐00764255]
Burk 2013 {published and unpublished data}
-
- Burks AW, Harthoorn LF, Langford JE, Ampting MT, Goldberg SB, Ong PY, et al. Functional effects of an amino‐acid based formula with synbiotics in cow's milk allergic infants. Allergy 2013;68(Suppl 97):703. [CENTRAL: CN‐00985742; EMBASE: 71369861]
Chernysov 2009 {published data only}
-
- Chernyshov PV. Randomized, placebo‐controlled trial on clinical and immunologic effects of probiotic and emollient in children with atopic eczema and cow milk allergy. Allergo Journal 2010;19(5):336‐7. [EMBASE: 70284293]
-
- Chernysov PV. Randomized, placebo‐controlled trial on clinical and immunologic effects of probiotic containing Lactobacillus rhamnosus R0011 and L. helveticus R0052 in infants with atopic dermatitis. Microbial Ecology in Health and Disease 2009;21(3‐4):228‐32. [EMBASE: 2010028537]
Foekel 2009 {published data only}
-
- Foekel C, Schubert R, Kaatz M, Schmidt I, Bauer A, Hipler UC, et al. Dietetic effects of oral intervention with mare's milk on the Severity Scoring of Atopic Dermatitis, on faecal microbiota and on immunological parameters in patients with atopic dermatitis. International Journal of Food Sciences and Nutrition 2009;60(Suppl 7):41‐52. [CENTRAL: CN‐00751360; PUBMED: 19462320] - PubMed
Gueniche 2008 {published data only}
-
- Gueniche A, Breton L. Vitreoscilla filiformis lysate, a probiotic profile for topical skin care. Journal of Investigative Dermatology 2009;129:S15. [EMBASE: 70384507]
-
- Gueniche A, Knaudt B, Schuck E, Volz T, Bastien P, Martin R, et al. Effects of nonpathogenic gram‐negative bacterium Vitreoscilla filiformis lysate on atopic dermatitis: a prospective, randomized, double‐blind, placebo‐controlled clinical study. British Journal of Dermatology 2008;159(6):1357‐63. [CENTRAL: CN‐00665626; PUBMED: 18795916] - PubMed
-
- Gueniche AG, Breton L. Vitreoscilla filiformis lysate, a probiotic profile for topical skin care. European Journal of Immunology 2009;39:S658. [EMBASE: 70347441]
Ikezawa 2004 {published data only}
-
- Ikezawa Z, Kondo M, Okajima M, Nishimura Y, Kono M. Clinical usefulness of oral itraconazole, an antimycotic drug, for refractory atopic dermatitis. European Journal of Dermatology 2004;14(6):400‐6. [CENTRAL: CN‐00504055; PUBMED: 15564204] - PubMed
Kalliomaki 2003 {published data only}
-
- Kalliomaki M, Salminen S, Poussa T, Arvilommi H, Isolauri E. Probiotics and prevention of atopic disease: 4‐year follow‐up of a randomised placebo controlled trial. Lancet 2003;361(9372):1869‐71. [CENTRAL: CN‐00558789; PUBMED: 12788576] - PubMed
Laitinen 2005 {published data only}
-
- Laitinen K, Kalliomaki M, Poussa T, Lagstrom H, Isolauri E. Evaluation of diet and growth in children with and without atopic eczema: follow‐up study from birth to 4 years. British Journal of Nutrition 2005;94(4):565‐74. [CENTRAL: CN‐00838395] - PubMed
Leung 2004 {published data only}
-
- Leung TF, Ma KC, Cheung LT, Lam CW, Wong E, Wan H, et al. A randomized, single‐blind and crossover study of an amino acid‐based milk formula in treating young children with atopic dermatitis. Pediatric Allergy and Immunology 2004;15(6):558‐61. [CENTRAL: CN‐00511281; PUBMED: 15610371] - PubMed
Matsumoto 2007 {published data only}
-
- Matsumoto M, Aranami A, Ishige A, Watanabe K, Benno Y. LKM512 yogurt consumption improves the intestinal environment and induces the T‐helper type 1 cytokine in adult patients with intractable atopic dermatitis. Clinical and Experimental Allergy 2007;37(3):358‐70. [CENTRAL: CN‐00641021; EMBASE: 2007128104] - PubMed
Moroi 2011 {published data only}
-
- Moroi M, Uchi S, Nakamura K, Sato S, Shimizu N, Fujii M, et al. Beneficial effect of a diet containing heat‐killed Lactobacillus paracasei K71 on adult type atopic dermatitis. Journal of Dermatology 2011;38(2):131‐9. [CENTRAL: CN‐00778680; PUBMED: 21269308] - PubMed
Murosaki 2006 {published data only}
-
- Murosaki S, Yamamoto Y, Yotsumoto H, Kubo H, Nomoto S, Usuki K, et al. Effects of intake of syrup supplemented with nigerooligosaccharides and heat‐killed Lactobacillus plantarum L‐137 on skin symptom and immune function in patients with atopic dermatitis. Japanese Pharmacology and Therapeutics 2006;34(10):1087‐96. [CENTRAL: CN‐00707197]
Ogawa 2006 {published data only}
-
- Ogawa T, Hashikawa S, Asai Y, Sakamoto H, Yasuda K, Makimura Y. A new synbiotic, Lactobacillus casei subsp. casei together with dextran, reduces murine and human allergic reaction. FEMS Immunology & Medical Microbiology 2006;46(3):400‐9. [CENTRAL: CN‐00563540; PUBMED: 16553814] - PubMed
Ou 2012 {published and unpublished data}
-
- Keet CA, Wood RA. Randomized, placebo‐controlled trial of Lactobacillus rhamnosus GG as treatment of atopic dermatitis in infancy. Pediatrics 2008;122(Suppl 4):S198‐9. [EMBASE: 2009166612]
-
- Ou C‐Y, Kuo H‐C, Wang L, Hsu T‐Y, Chuang H, Liu C‐A, et al. Prenatal and postnatal probiotics reduces maternal but not childhood allergic diseases: a randomized, double‐blind, placebo‐controlled trial. Clinical and Experimental Allergy 2012;42(9):1386‐96. [CENTRAL: CN‐00850372; EMBASE: 2012510284; PUBMED: 22925325] - PubMed
Rose 2010 {published and unpublished data}
-
- Rose MA, Schubert R, Schulze J, Zielen S. Follow‐up of probiotic Lactobacillus GG effects on allergic sensitization and asthma in infants at risk. Clinical and Experimental Allergy 2011;41(12):1819‐21. [CENTRAL: CN‐00834541] - PubMed
-
- Rose MA, Stieglitz F, Köksal A, Schubert R, Schulze J, Zielen S. Efficacy of probiotic Lactobacillus GG on allergic sensitization and asthma in infants at risk. Clinical and Experimental Allergy 2010;40(9):1398‐405. [CENTRAL: CN‐00767900; PUBMED: 20604800] - PubMed
Shibata 2009 {published data only}
-
- Shibata R, Kimura M, Takahashi H, Mikami K, Aiba Y, Takeda H, et al. Clinical effects of kestose, a prebiotic oligosaccharide, on the treatment of atopic dermatitis in infants. Clinical and Experimental Allergy 2009;39(9):1397‐403. [CENTRAL: CN‐00719173; PUBMED: 19508323] - PubMed
-
- Shibata R, Koga Y, Takeda H, Nishima S. Clinical effects of prebiotics with kestose, a fructo‐oligosaccharide, on atopic dermatitis in infants. Allergy 2009;64:447. [CENTRAL: CN‐01029517] - PubMed
Torii 2011 {published data only}
-
- Torii S, Torii A, Itoh K, Urisu A, Terada A, Fujisawa T, et al. Effects of oral administration of Lactobacillus acidophilus L‐92 on the symptoms and serum markers of atopic dermatitis in children. International Archives of Allergy and Immunology 2011;154(3):236‐45. [CENTRAL: CN‐00779204; PUBMED: 20861645] - PubMed
References to studies awaiting assessment
ACTRN12605000615684 {published data only}
-
- ACTRN12605000615684. Probiotics in the management of eczema [A double‐blind, randomised, placebo controlled study to identify markers for the differential response of children with eczema following treatment with probiotics]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=782 (first received 23 September 2005).
Candy 2016 {published data only}
-
- Candy DCA, Ampting MTJ, Oude Nijhuis MM, Wopereis H, Butt AM, Peroni DG, et al. Dietary management of non‐IgE mediated cow's milk allergic infants with a synbiotics‐supplemented amino acid‐based formula: effects on faecal microbiota and clinical symptoms. Journal of Pediatric Gastroenterology, Hepatology and Nutrition. Lippincott Williams and Wilkins, 2016:S402.
Hulshof 2017 {published data only}
-
- Hulshof L, Overbeek SA, Wyllie Al, Chu Mj, Bogaert D, Jager W, et al. Dietary intervention with a synbiotic mixture of scGOS/lcFOS with Bifidobacterium breve M‐16V in infants with atopic dermatitis shows beneficial immunological changes in chemokine profiles. Allergy: European Journal of Allergy and Clinical Immunology 2017;72:309‐10. [CENTRAL: CN‐01417584]
NCT02585986 {published data only}
-
- NCT02585986. Intervention study to evaluate a probiotic in mild atopic dermatitis young patients [Randomized, double blind, placebo‐controlled Intervention study to evaluate safety and efficiency of a probiotic in symptoms reduction and use of topic corticoids in mild atopic dermatitis patients aged 4 to 17 years]. clinicaltrials.gov/ct2/show/NCT02585986 (first received 13 October 2015).
Prakoeswa 2017 {published data only}
-
- Prakoeswa CRS, Herwanto N, Prameswari R, Astari L, Sawitri S, Hidayati AN, et al. Lactobacillus plantarum IS‐10506 supplementation reduced SCORAD in children with atopic dermatitis. Beneficial Microbes 2017;8(5):833‐40. [CENTRAL: CN‐01420752] - PubMed
References to ongoing studies
ChiCTR1800015330 {published data only}
-
- ChiCTR1800015330. Effects of probiotics on inflammation and intestinal microflora in patients with atopic dermatitis. www.chictr.org.cn/showprojen.aspx?proj=24603 (first received 23 March 2018).
CTRI/2017/08/009236 {published data only}
-
- CTRI/2017/08/009236. A study to observe if probiotics supplementation is helpful in atopic dermatitis children. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2017/08/009236 (first received 2 August 2018).
CTRI/2017/10/010018 {published data only}
-
- CTRI/2017/10/010018. Probiotics in treatment of atopic dermatitis in children. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2017/10/010018 (first received 6 October 2017).
KCT0000914 {published data only}
-
- KCT0000914. A 8 week, randomized, double‐blind, placebo‐controlled clinical trial for the evaluation of the efficacy and safety of IRT5 probiotics on atopic dermatitis in children [IRT5 probiotics atopic dermatitis]. cris.nih.go.kr/cris/en/search/search_result_st01.jsp?seq=3350 (accessed before 7 December 2016).
NCT02519556 {published data only}
-
- NCT02519556. Trial on effectiveness combined probiotics in atopic dermatitis in children [Randomized, double blind, controlled trial on effectiveness combined probiotics in the treatment of atopic dermatitis in children up to 6 months]. clinicaltrials.gov/ct2/show/NCT02519556 (first received 4 August 2015).
NCT02945683 {published data only}
-
- NCT02945683. Effects of Lactobacillus reuteri plus vitamin D3 in children with atopic dermatitis. clinicaltrials.gov/ct2/show/NCT02945683 (first received 26 October 2016).
Additional references
Abrahamsson 2012
-
- Abrahamsson TR, Jakobsson HE, Andersson AF, Björksten B, Engstrand L, Jenmalm MC. Low diversity of the gut microbiota in infants with atopic eczema. Journal of Allergy and Clinical Immunology 2012;129(2):434‐40. [PUBMED: 22153774] - PubMed
Abuabara 2018
Allen 2003
Allingstrup 2016
Atkins 2004
Basra 2007
-
- Basra MK, Sue‐Ho R, Finlay AY. The Family Dermatology Life Quality Index: measuring the secondary impact of skin disease. British Journal of Dermatology 2007;156(4):791. [PUBMED: 17300244] - PubMed
Beattie 2006
Beck 2000
-
- Beck LA, Leung DY. Allergen sensitization through the skin induces systemic allergic responses. Journal of Allergy & Clinical Immunology 2000;106(5 Suppl):S258‐63. [PUBMED: 11080741] - PubMed
Besselink 2008
-
- Besselink MG, Santvoort HC, Buskens E, Boermeester MA, Goor H, Timmerman HM, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised double‐blind placebo‐controlled trial. Lancet 2008;371(9613):651‐9. [PUBMED: 18279948 ] - PubMed
Bieber 2008
-
- Bieber T. Atopic dermatitis. New England Journal of Medicine 2008;358(14):1483‐94. [PUBMED: 18385500] - PubMed
Bjorksten 2001
-
- Bjorksten B, Sepp E, Julge K, Voor T, Mikelsaar M. Allergy development and the intestinal microflora during the first year of life. Journal of Allergy and Clinical Immunology 2001;108(4):516‐20. [PUBMED: 11590374] - PubMed
Bohme 2001
-
- Bohme M, Svensson A, Kull I, Nordvall SL, Wahlgren CF. Clinical features of atopic dermatitis at two years of age: a prospective, population‐based case‐control study. Acta Dermato‐Venereologica 2001;81(3):193‐7. [PUBMED: 11558876] - PubMed
Borriello 2003
-
- Borriello SP, Hammes WP, Holzapfel W, Marteau P, Schrezenmeir J, Vaara M, et al. Safety of probiotics that contain lactobacilli or bifidobacteria. Clinical Infectious Diseases 2003;36(6):775‐80. [PUBMED: 12627362] - PubMed
Boyle 2006a
-
- Boyle RJ, Robins‐Browne RM, Tang ML. Probiotic use in clinical practice: what are the risks?. American Journal of Clinical Nutrition 2006;83(6):1256‐64. [PUBMED: 16762934] - PubMed
Brok 2008
-
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61(8):763‐9. [PUBMED: 18411040] - PubMed
Brok 2009
-
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive ‐ trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [PUBMED: 18824466] - PubMed
Burton 2006
Caffarelli 1998
Chang 2016a
Charman 2002
-
- Charman C, Williams HC. Epidemiology. In: Thomas Bieber, Donald YM Leung editor(s). Atopic Dermatitis. 1st Edition. New York: Marcel Dekker Inc, 2002:21‐42.
Cherifi 2004
-
- Cherifi S, Robberecht J, Miendje Y. Saccharomyces cerevisiae fungemia in an elderly patient with Clostridium difficile colitis. Acta Clinica Belgica 2004;59(4):223‐4. [PUBMED: 15597730] - PubMed
Chren 1997
-
- Chren MM, Lasek RJ, Flocke SA, Zyzanski SJ. Improved discriminative and evaluative capability of a refined version of SKINDEX, a quality‐of‐life instrument for patients with skin diseases. Archives of Dermatology 1997;133(11):1433‐40. [PUBMED: 9371029] - PubMed
Christensen 2002
-
- Christensen HR, Frokiaer H, Pestka JJ. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. Journal of Immunology 2002;168(1):171‐8. [PUBMED: 11751960] - PubMed
Connolly 2005
-
- Connolly E, Abrahamsson T, Bjorksten B. Safety of D(‐)‐lactic acid producing bacteria in the human infant. Journal of Pediatric Gastroenterology & Nutrition 2005;41(4):489‐92. [PUBMED: 16205524] - PubMed
Dang 2013
-
- Dang D, Zhou W, Lun ZJ, Mu X, Wang DX, Wu H. Meta‐analysis of probiotics and/or prebiotics for the prevention of eczema. Journal of International Medical Research 2013;41(5):1426‐36. [PUBMED: 23908398] - PubMed
De Groote 2005
-
- Groote MA, Frank DN, Dowell E, Glode MP, Pace NR. Lactobacillus rhamnosus GG bacteremia associated with probiotic use in a child with short gut syndrome. Pediatric Infectious Disease Journal 2005;24(3):278‐80. [PUBMED: 15750472] - PubMed
Didari 2014
-
- Didari T, Solki S, Mozaffari S, Nikfar S, Abdollahi M. A systematic review of the safety of probiotics. Expert Opinion on Drug Safety 2014;13(2):227‐39. [PUBMED: 24405164] - PubMed
Diepgen 1996
-
- Diepgen TL, Sauerbrei W, Fartasch M. Development and validation of diagnostic scores of atopic dermatitis incorporating criteria of data quality and practical usefulness. Journal of Clinical Epidemiology 1996;49:1031‐8. - PubMed
Doege 2012
-
- Doege K, Grajecki D, Zyrax BC, Dentinika E, Zu Eulenburg C, Buhling KJ. Impact of maternal supplementation with probiotics during pregnancy on atopic eczema in childhood ‐ a meta‐analysis. British Journal of Nutrition 2012;107(1):1‐6. [PUBMED: 21787448] - PubMed
Duffy 1989
-
- Duffy SW, Rohan TE, Altman DG. A method for combining matched and unmatched binary data. Application to randomized, controlled trials of photocoagulation in the treatment of diabetic retinopathy. American Journal of Epidemiology 1989;130(2):371‐8. [PUBMED: 2750732] - PubMed
Dugoua 2009
-
- Dugoua JJ, Machado MM, Zhu X, Chen X, Koren G, Einarson T. Probiotic safety in pregnancy: a systematic review and meta‐analysis of randomized controlled trials of Lactobacillus, Bifidobacterium and Saccharomyces spp. Journal of Obstetrics and Gynaecology Canada 2009;31(6):542‐52. [PUBMED: 19646321] - PubMed
Eichenfield 2004
-
- Eichenfield LF. Consensus guidelines in diagnosis and treatment of atopic dermatitis. Allergy 2004;59(Suppl 78):86‐92. [PUBMED: 15245365] - PubMed
Eichenfield 2017
-
- Eichenfield LF, Stein Gold LF. Addressing the immunopathogenesis of atopic dermatitis: advances in topical and systemic treatment. Seminars in Cutaneous Medicine and Surgery 2017;36(2 Suppl 2):S45‐8. [PUBMED: 28654711] - PubMed
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vaillancourt JM. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [PUBMED: 11914310] - PubMed
Ellis 2002
-
- Ellis CN, Drake LA, Prendergast MM, Abramovits W, Boguniewicz M, Daniel CR, et al. Cost of atopic dermatitis and eczema in the United States. Journal of the American Academy of Dermatology 2002;46(3):361‐70. [PUBMED: 11862170] - PubMed
Enomoto 2008
-
- Enomoto H, Hirata K, Otsuka K, Kawai T, Takahashi T, Hirota T, et al. Filaggrin null mutations are associated with atopic dermatitis and elevated levels of IgE in the Japanese population: a family and case‐control study. Journal of Human Genetics 2008;53(7):615‐21. [PUBMED: 18521703] - PubMed
Ernst 2000
-
- Ernst E, Pittler MH, Stevinson C. Complementary/alternative medicine in dermatology: evidence‐assessed efficacy of two diseases and two treatments. American Journal of Clinical Dermatology 2000;3(5):341‐8. [PUBMED: 12069640] - PubMed
FAO/WHO 2002
-
- Joint FAO/WHO Working Group. Guidelines for the evaluation of probiotics in food 2002. www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf?ua=1. London, Ontario, Canada, (accessed before 8 December 2016).
Fennessy 2000
-
- Fennessy M, Coupland S, Popay J, Naysmith K. The epidemiology and experience of atopic eczema during childhood: a discussion paper on the implications of current knowledge for health care, public health policy and research. Journal of Epidemiology & Community Health 2000;54(8):581‐9. [PUBMED: 10890869] - PMC - PubMed
Finlay 1996
-
- Finlay AY. Measurement of disease activity and outcome in atopic dermatitis. British Journal of Dermatology 1996;135(4):509‐15. [PUBMED: 8915137] - PubMed
Fiocchi 2012
Flohr 2004
-
- Flohr C, Johansson SG, Wahlgren CF, Williams H. How atopic is atopic dermatitis?. Journal of Allergy and Clinical Immunology 2004;114(1):150‐8. [PUBMED: 15241359] - PubMed
Guarino 2015
-
- Guarino A, Guandalini S, Lo Vecchio A. Probiotics for the prevention and treatment for diarrhea. Journal of Clinical Gastroenterology 2015;49(Suppl 1):S37‐45. [PUBMED: 26447963] - PubMed
Hempel 2011
-
- Hempel S, Newberry S, Ruelaz A, Wang Z, Miles JNV, Suttorp MJ, et al. Safety of probiotics to reduce risk and prevent or treat disease. Evidence Report/ Technology Assessment Number 200. AHRQ Publication No. 11‐E007. April 2011. www.ahrq.gov/sites/default/files/wysiwyg/research/findings/evidence‐base... (accessed before 8 December 2016).
Hennequin 2000
-
- Hennequin C, Kauffmann‐Lacroix C, Jobert A, Viard JP, Ricour C, Jacquemin JL, et al. Possible role of catheters in Saccharomyces boulardii fungemia. European Journal of Clinical Microbiology and Infectious Diseases 2000;19(1):16‐20. [PUBMED: 10706174] - PubMed
Higgins 2011
-
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. The Cochrane Collaboration. Available from www.cochrane‐handbook.org.
Hoare 2000
Holzapfel 2001
-
- Holzapfel WH, Haberer P, Geisen R, Bjorkroth J, Schillinger U. Taxonomy and important features of probiotic microorganisms in food and nutrition. American Journal of Clinical Nutrition 2001;73(2 Suppl):365S‐73S. [PUBMED: 11157343] - PubMed
Huang 2017
-
- Huang R, Ning H, Shen M, Li J, Zhang J, Chen X. Probiotics for the treatment of atopic dermatitis in children: a systematic review and meta‐analysis of randomized controlled trials. Frontiers in Cellular and Infection Microbiology 2017;7:392. [DOI: 10.3389/fcimb.2017.00392; PUBMED: 28932705] - DOI - PMC - PubMed
Ishibashi 2001
-
- Ishibashi N, Yamazaki S. Probiotics and safety. American Journal of Clinical Nutrition 2001;73(2 Suppl):S465‐70. [PUBMED: 11157359] - PubMed
Ismail 2012
-
- Ismail IH, Oppedisano F, Joseph SJ, Boyle RJ, Licciardi PV, Robins‐Browne RM, et al. Reduced gut microbial diversity in early life is associated with later development of eczema but not atopy in high risk infants. Paediatric Allergy and Immunology 2012;23(7):674‐81. [PUBMED: 22831283] - PubMed
Johansson 2001
-
- Johansson SG, Hourihane JO, Bousquet J, Bruijnzeel‐Koomen C, Dreborg S, Haahtela T, et al. A revised nomenclature for allergy: an EAACI position statement from the EAACI nomenclature task force. Allergy 2001;56(9):813‐24. [PUBMED: 11551246] - PubMed
Johansson 2004
-
- Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, et al. Revised nomenclature for allergy for global use: report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. Journal of Allergy and Clinical Immunology 2004;113(5):832‐6. [PUBMED: 15131563] - PubMed
Juni 2001
Kalliomaki 2001
-
- Kalliomaki M, Kirjavainen P, Eerola E, Kero P, Salminen S, Isolauri E. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. Journal of Allergy and Clinical Immunology 2001;107(1):129‐34. [PUBMED: 11150002] - PubMed
Kemp 1999
-
- Kemp AS. Atopic eczema: its social and financial costs. Journal of Paediatrics and Child Health 1999;35(3):229‐31. [PUBMED: 10404440] - PubMed
Kim 2014
-
- Kim SO, Ah YM, Yu YM, Choi KH, Shin WG, Lee JY. Effects of probiotics for the treatment of atopic dermatitis: a meta‐analysis of randomized controlled trials. Annals of Allergy, Asthma, & Immunology 2014;113(2):217‐26. [PUBMED: 24954372] - PubMed
Kukkonen 2007
-
- Kukkonen K, Savilahti E, Haahtela T, Juntunen‐Backman K, Korpela R, Poussa T, et al. Probiotics and prebiotic galacto‐oligosaccharides in the prevention of allergic diseases: a randomized, double‐blind, placebo‐controlled trial. Journal of Allergy and Clinical Immunology 2007;119(1):192‐8. [PUBMED: 17208601] - PubMed
Kunz 1997
-
- Kunz B, Oranje AP, Labreze L, Stalder JF, Ring J, Taieb A. Clinical validation and guidelines for the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology 1997;195(1):10‐9. [PUBMED: 9267730] - PubMed
Lamb 2002
-
- Lamb SR, Rademaker M. Pharmacoeconomics of drug therapy for atopic dermatitis. Expert Opinion on Pharmacotherapy 2002;3(3):249‐55. [PUBMED: 11866675] - PubMed
Land 2005
-
- Land MH, Rouster‐Stevens K, Woods CR, Cannon ML, Cnota J, Shetty AK. Lactobacillus sepsis associated with probiotic therapy. Pediatrics 2005;115(1):178‐81. [PUBMED: 15629999] - PubMed
Lawson 1998
-
- Lawson V, Lewis‐Jones MS, Finlay AY, Reid P, Owens RG. The family impact of childhood atopic dermatitis: the Dermatitis Family Impact Questionnaire. British Journal of Dermatology 1998;138(1):107‐13. [PUBMED: 9536231] - PubMed
Lestin 2003
-
- Lestin F, Pertschy A, Rimek D. Fungemia after oral treatment with Saccharomyces boulardii in a patient with multiple comorbidities [Fungamie nach oraler Gabe]. Deutsche Medizinische Wochenschrift 2003;128(48):2531‐3. [PUBMED: 14648435] - PubMed
Lewis‐Jones 2006
Makelainen 2003
-
- Makelainen H, Tahvonen R, Salminen S, Ouwehand AC. In vivo safety assessment of two Bifidobacterium longum strains. Microbiology & Immunology 2003;47(12):911‐4. [PUBMED: 14695440] - PubMed
Malik 2017
-
- Malik K, Heitmiller KD, Czarnowski T. An update on the pathophysiology of atopic dermatitis. Dermatologic Clinics 2017;35(3):317‐26. [PUBMED: 28577801] - PubMed
Mancini 2008
-
- Mancini AJ, Kaulback K, Chamlin SL. The socioeconomic impact of atopic dermatitis in the United States: a systematic review. Pediatric Dermatology 2008;25(1):1‐6. [PUBMED: 18304144] - PubMed
Mansfield 2014
-
- Mansfield JA, Bergin SW, Cooper JR, Olsen CH. Comparative probiotic strain efficacy in the prevention of eczema in infants and children: a systematic review and meta‐analysis. Military Medicine 2014;179(6):580‐92. [PUBMED: 24902123] - PubMed
Moro 2006
Murray 2005
-
- Murray CS, Tannock GW, Simon MA, Harmsen HJ, Welling GW, Custovic A, et al. Fecal microbiota in sensitized wheezy and non‐sensitized non‐wheezy children: a nested case‐control study. Clinical and Experimental Allergy 2005;35(6):741‐5. [PUBMED: 15969664] - PubMed
Nankervis 2016
-
- Nankervis H, Thomas KS, Delamere FM, Barbarot S, Rogers NK, Williams HC. Scoping Systematic Review of Treatments for Eczema. 7th Edition. Vol. 4, Programme Grants for Applied Research, NIHR Journals Library, 2016. [PUBMED: 27280278] - PubMed
Nemoto‐Hasebe 2009
-
- Nemoto‐Hasebe I, Akiyama M, Nomura T, Sandilands A, McLean WH, Shimizu H. FLG mutation p.Lys4021X in the C‐terminal imperfect filaggrin repeat in Japanese patients with atopic eczema. British Journal of Dermatology 2009;161(6):1387‐90. [PUBMED: 19663875] - PubMed
Odhiambo 2009
-
- Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher IM, ISAAC Phase Three Study Group. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. Journal of Allergy and Clinical Immunology 2009;124(6):1251‐8. [PUBMED: 20004783] - PubMed
Osborn 2007
Palmer 2006
-
- Palmer CN, Irvine AD, Terron‐Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss‐of‐function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nature Genetics 2006;38(4):441‐6. [PUBMED: 16550169] - PubMed
Pineiro 2007
-
- Pineiro M, Stanton C. Probiotic bacteria: legislative framework ‐ requirements to evidence basis. Journal of Nutrition 2007;137(3 Suppl 2):850S‐3S. [PUBMED: 17311986] - PubMed
Riquelme 2003
-
- Riquelme AJ, Calvo MA, Guzman AM, Depix MS, Garcia P, Perez C, et al. Saccharomyces cerevisiae fungemia after Saccharomyces boulardii treatment in immunocompromised patients. Journal of Clinical Gastroenterology 2003;36(1):41‐3. [PUBMED: 12488707] - PubMed
Rook 2016
-
- Arden‐Jones MR, Flohr C, Reynolds NJ, Holden CA. Atopic eczema. In: Griffiths CE, Barker J, Bleiker T, Chalmers R, Creamer D editor(s). Rook's Textbook of Dermatology. 9th Edition. Vol. 2, New York, NY: John Wiley & Sons, 2016.
Rosenfeldt 2004
-
- Rosenfeldt V, Benfeldt E, Valerius NH, Paerregaard A, Michaelsen KF. Effect of probiotics on gastrointestinal symptoms and small intestinal permeability in children with atopic dermatitis. Journal of Pediatrics 2004;145(5):612‐6. [PUBMED: 15520759] - PubMed
Ruden 1999
-
- Ruden U, Staab D, Kehrt R, Wahn U. Development and validation of a disease‐specific questionnaire to measure quality of life in parents of children with atopic dermatitis [Entwicklung und Validierung eines krankheitsspezifischen Fragebogens zur Erfassung der Lebensqualitat von Eltern neurodermitiskranker Kinder]. Zeitschrift fur Gesundheitswissenschaften 1999;7:335‐50. [DOI: 10.1007/BF02966499] - DOI
Salminen 1998
-
- Salminen S, Wright A, Morelli L, Marteau P, Brassart D, Vos WM, et al. Demonstration of safety of probiotics ‐ a review. International Journal of Food Microbiology 1998;44(1‐2):93‐106. [PUBMED: 9849787] - PubMed
Schmitt 2013
-
- Schmitt J, Langan S, Deckert S, Svensson A, Kobyletzki L, Thomas K, et al. Assessment of clinical signs of atopic dermatitis: a systematic review and recommendation. Journal of Allergy and Clinical Immunology 2013;132(6):1337‐47. [PUBMED: 24035157] - PubMed
Schram 2011
-
- Schram ME, Spuls PI, Leeflang MM, Lindeboom R, Bos JD, Schmitt J. EASI, (objective) SCORAD and POEM for atopic eczema: responsiveness and minimal clinically important difference. Allergy 2012;67(1):99‐106. [PUBMED: 21951293] - PubMed
Silverberg 2013
-
- Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population‐based study. Journal of Allergy and Clinical Immunology 2013;132(5):1132‐8. [PUBMED: 24094544] - PubMed
Silverberg 2017
Simonyte Sjödin 2016
-
- Simonyte Sjödin K, Vidman L, Rydén P, West CE. Emerging evidence of the role of gut microbiota in the development of allergic diseases. Current Opinion in Allergy and Clinical Immunology 2016;16(4):390‐5. [PUBMED: 27253486] - PubMed
Smethurst 2002
-
- Smethurst D. Atopic eczema. Clinical Evidence. 8th Edition. London: BMJ, 2002:1664‐82. - PubMed
Song 2016
-
- Song H, Yoo Y, Hwang J, Na YC, Kim HS. Faecalibacterium prausnitzii subspecies‐level dysbiosis in the human gut microbiome underlying atopic dermatitis. Journal of Allergy and Clinical Immunology 2016;137(3):852‐60. [PUBMED: 26431583] - PubMed
Srinivasan 2006
-
- Srinivasan R, Meyer R, Padmanabhan R, Britto J. Clinical safety of Lactobacillus casei shirota as a probiotic in critically ill children. Journal of Pediatric Gastroenterology & Nutrition 2006;42(2):171‐3. [PUBMED: 16456410] - PubMed
TSA software [Computer program]
-
- Copenhagen Trial Unit, Center for Clinical Intervention Research, Rigshospitalet. Trial Sequential Analysis (TSA). Version 0.9.5.10 Beta. Copenhagen, Denmark: Copenhagen Trial Unit, Center for Clinical Intervention Research, Rigshospitalet, 2017.
Vitaliti 2014
-
- Vitaliti G, Pavone P, Guglielmo F, Spataro G, Falsaperla R. The immunomodulatory effect of probiotics beyond atopy: an update. Journal of Asthma 2014;51(3):320‐32. [PUBMED: 24256057] - PubMed
Wang 2008
-
- Wang M, Karlsson C, Olsson C, Adlerberth I, Wold AE, Strachan DP, et al. Reduced diversity in the early fecal microbiota of infants with atopic eczema. Journal of Allergy and Clinical Immunology 2008;121(1):129‐34. [PUBMED: 18028995] - PubMed
Watanabe 2003
-
- Watanabe S, Narisawa Y, Arase S, Okamatsu H, Ikenaga T, Tajiri Y, et al. Differences in fecal microflora between patients with atopic dermatitis and healthy control subjects. Journal of Allergy and Clinical Immunology 2003;111(3):587‐91. [PUBMED: 12642841] - PubMed
Wetterslev 2008
-
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [PUBMED: 18083463] - PubMed
Wetterslev 2009
Williams 1999
-
- Williams H, Robertson C, Stewart A, Ait‐Khaled N, Anabwani G, Anderson R, et al. Worldwide variations in the prevalence of symptoms of atopic eczema in the International Study of Asthma and Allergies in Childhood. Journal of Allergy and Clinical Immunology 1999;103(1 Pt 1):125‐38. [PUBMED: 9893196] - PubMed
Williams 2005
-
- Williams HC. Clinical practice. Atopic dermatitis. New England Journal of Medicine 2005;352(22):2314‐24. [PUBMED: 15930422] - PubMed
Williams 2008
-
- Williams H, Stewart A, Mutius E, Cookson W, Anderson RH, International Study of Asthma and Allergies in Childhood (ISAAC) Phase One and Three Study Groups. Is eczema really on the increase worldwide. Journal of Allergy and Clinical Immunology 2008;121(4):947‐54. [PUBMED: 18155278] - PubMed
Wolf 1998
-
- Wolf BW, Wheeler KB, Ataya DG, Garleb KA. Safety and tolerance of Lactobacillus reuteri supplementation to a population infected with the human immunodeficiency virus. Food & Chemical Toxicology 1998;36(12):1085‐94. [PUBMED: 9862651] - PubMed
Zhang 2011
-
- Zhang H, Guo Y, Wang W, Shi M, Chen X, Yao Z. Mutations in the filaggrin gene in Han Chinese patients with atopic dermatitis. Allergy 2011;66(3):420‐7. [PUBMED: 21039602] - PubMed
Zhu 2010
-
- Zhu DL, Yang WX, Yang HM. Meta analysis of lactic acid bacteria as probiotics for the primary prevention of infantile eczema. Zhonqquo Dang Dai Er Ke Za Zhi 2010;12(9):734‐9. [PUBMED: 20849726] - PubMed
References to other published versions of this review
Boyle 2006b
-
- Boyle RJ, Bath‐Hextall F, Donath S, Murrell D, Tang MLK, Taylor J, et al. Probiotics for atopic eczema. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD006135] - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

