Anti-angiogenic therapy for high-grade glioma
- PMID: 30480778
- PMCID: PMC6516839
- DOI: 10.1002/14651858.CD008218.pub4
Anti-angiogenic therapy for high-grade glioma
Abstract
Background: This is an updated version of the original Cochrane Review published in September 2014. The most common primary brain tumours in adults are gliomas. Gliomas span a spectrum from low to high grade and are graded pathologically on a scale of one to four according to the World Health Organization (WHO) classification. High-grade glioma (HGG) carries a poor prognosis. Grade IV glioma is known as glioblastoma and carries a median survival in treated patients of about 15 months. Glioblastomas are rich in blood vessels (i.e. highly vascular) and also rich in a protein known as vascular endothelial growth factor (VEGF) that promotes new blood vessel formation (the process of angiogenesis). Anti-angiogenic agents inhibit the process of new blood vessel formation and promote regression of existing vessels. Several anti-angiogenic agents have been investigated in clinical trials, both in newly diagnosed and recurrent HGG, showing preliminary promising results. This review was undertaken to report on the benefits and harms associated with the use of anti-angiogenic agents in the treatment of HGGs.
Objectives: To evaluate the efficacy and toxicity of anti-angiogenic therapy in people with high-grade glioma (HGG). The intervention can be used in two broad groups: at first diagnosis as part of 'adjuvant' therapy, or in the setting of recurrent disease.
Search methods: We conducted updated searches to identify published and unpublished randomised controlled trials (RCTs), including the Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 9), MEDLINE and Embase to October 2018. We handsearched proceedings of relevant oncology conferences up to 2018. We also searched trial registries for ongoing studies.
Selection criteria: RCTs evaluating the use of anti-angiogenic therapy to treat HGG versus the same therapy without anti-angiogenic therapy.
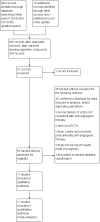
Data collection and analysis: Review authors screened the search results and reviewed the abstracts of potentially relevant articles before retrieving the full text of eligible articles.
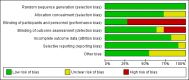
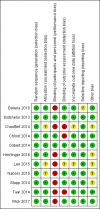
Main results: After a comprehensive literature search, we identified 11 eligible RCTs (3743 participants), of which 7 were included in the original review (2987 participants). There was significant design heterogeneity in the included studies, especially in the response assessment criteria used. All eligible studies were restricted to glioblastomas and there were no eligible studies evaluating other HGGs. Ten studies were available as fully published peer-reviewed manuscripts, and one study was available in abstract form. The overall risk of bias in included studies was low. This risk was based upon low rates of selection bias, detection bias, attrition bias and reporting bias. The 11 studies included in this review did not show an improvement in overall survival with the addition of anti-angiogenic therapy (pooled hazard ratio (HR) of 0.95, 95% confidence interval (CI) 0.88 to 1.02; P = 0.16; 11 studies, 3743 participants; high-certainty evidence). However, pooled analysis from 10 studies (3595 participants) showed improvement in progression-free survival with the addition of anti-angiogenic therapy (HR 0.73, 95% CI 0.68 to 0.79; P < 0.00001; high-certainty evidence).We carried out additional analyses of overall survival and progression-free survival according to treatment setting and for anti-angiogenic therapy combined with chemotherapy compared to chemotherapy alone. Pooled analysis of overall survival in either the adjuvant or recurrent setting did not show an improvement (HR 0.93, 95% CI 0.86 to 1.02; P = 0.12; 8 studies, 2833 participants; high-certainty evidence and HR 0.99, 95% CI 0.85 to 1.16; P = 0.90; 3 studies, 910 participants; moderate-certainty evidence, respectively). Pooled analysis of overall survival for anti-angiogenic therapy combined with chemotherapy compared to chemotherapy also did not clearly show an improvement (HR 0.92, 95% CI 0.85 to 1.00; P = 0.05; 11 studies, 3506 participants; low-certainty evidence). The progression-free survival in the subgroups all showed findings that demonstrated improvements in progression-free survival with the addition of anti-angiogenic therapy. Pooled analysis of progression-free survival in both the adjuvant and recurrent setting showed an improvement (HR 0.75, 95% CI 0.69 to 0.82; P < 0.00001; 8 studies, 2833 participants; high-certainty evidence and HR 0.64, 95% CI 0.54 to 0.76; P < 0.00001; 2 studies, 762 participants; moderate-certainty evidence, respectively). Pooled analysis of progression-free survival for anti-angiogenic therapy combined with chemotherapy compared to chemotherapy alone showed an improvement (HR 0.72, 95% CI 0.66 to 0.77; P < 0.00001; 10 studies, 3464 participants). Similar to trials of anti-angiogenic therapies in other solid tumours, adverse events related to this class of therapy included hypertension and proteinuria, poor wound healing, and the potential for thromboembolic events, although generally, the rate of grade 3 and 4 adverse events was low (< 14.1%) and in keeping with the literature. The impact of anti-angiogenic therapy on quality of life varied between studies.
Authors' conclusions: The use of anti-angiogenic therapy does not significantly improve overall survival in newly diagnosed people with glioblastoma. Thus, there is insufficient evidence to support the use of anti-angiogenic therapy for people with newly diagnosed glioblastoma at this time. Overall there is a lack of evidence of a survival advantage for anti-angiogenic therapy over chemotherapy in recurrent glioblastoma. When considering the combination anti-angiogenic therapy with chemotherapy compared with the same chemotherapy alone, there may possibly be a small improvement in overall survival. While there is strong evidence that bevacizumab (an anti-angiogenic drug) prolongs progression-free survival in newly diagnosed and recurrent glioblastoma, the impact of this on quality of life and net clinical benefit for patients remains unclear. Not addressed here is whether subsets of people with glioblastoma may benefit from anti-angiogenic therapies, nor their utility in other HGG histologies.
Conflict of interest statement
Nick Pavlakis ‐ none known
Helen Wheeler ‐ the analysis for this Cochrane Review is based on peer‐reviewed data which was prepared by an independent steering trials committee. My involvement in the Australian Roche Advisory Board was to discuss completed trial results and how the drug may be introduced into the clinic in Australian centres. My participation on the merck serono centric steering committee was to review ongoing trial recruitment and serious adverse events. None of these activities influenced the analysis of the review data or contributed to any presented/published conclusions.
Robin Grant ‐ no conflict of interest related to this review
John Simes ‐ I have no relevant conflicts of interest to declare. My institution has received research funding support from Merck KGa and Roche.
Malaka Ameratunga‐ none known
Mustafa Khasraw ‐ none known. My institution has received research funding support from Merck KGa and I have served on glioblastoma advisory boards of Roche.
Figures









Update of
-
Antiangiogenic therapy for high-grade glioma.Cochrane Database Syst Rev. 2014 Sep 22;(9):CD008218. doi: 10.1002/14651858.CD008218.pub3. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2018 Nov 22;11:CD008218. doi: 10.1002/14651858.CD008218.pub4. PMID: 25242542 Updated.
References
References to studies included in this review
Balana 2016 {published data only}
-
- Balana C, Las Penas R, Sepulveda JM, Gil‐Gil MJ, Luque R, Gallego O, et al. Bevacizumab and temozolomide versus temozolomide alone as neoadjuvant treatment in unresected glioblastoma:the GENOM 009 randomized phase II trial. Journal of Neurooncology 2016;127:569‐79. [DOI: 10.1007/s11060-016-2065-5] - DOI - PubMed
Batchelor 2013 {published data only}
-
- Batchelor TT, Mulholland P, Neyns B, Nabors LB, Campone M, Wick A, et al. Phase III randomised trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. Journal of Clinical Oncology 2013;31(26):3212‐8. - PMC - PubMed
Chauffert 2014 {published data only}
-
- Chauffert B, Feuvret L, Bonnetain F, Taillandier L, Frappaz D, Taillia H, et al. Randomized phase II trial of irinotecan and bevacizumab as neo‐adjuvant and adjuvant to temozolomide‐based chemoradiation compared with temozolomide‐chemoradiation for unresectable glioblastoma: final results of the TEMAVIR study from ANOCEF. Annals of Oncology 2014;25:1442‐7. [DOI: 10.1093/annonc/mdu148] - DOI - PubMed
Chinot 2014 {published data only}
-
- Sandmann T, Bourgon R, Garcia J, Li C, Cloughesy T, Chinot Ol, et al. Patients with proneural glioblastoma may derive overall survival benefit from the addition of bevacizumab to first‐line radiotherapy and temozolomide: retrospective analysis of the AVAglio Trial. Journal of Clinical Oncology 2015;33(25):2735‐44. - PMC - PubMed
-
- Taphoorn MJ, Henriksson R, Bottomley A, Cloughesy T, Wick W, Mason WP, et al. Health‐related quality of life in a randomized phase III study of bevacizumab, temozolomide, and radiotherapy in newly diagnosed glioblastoma.. Journal of Clinical Oncology 2015;33(19):2166‐75. - PubMed
Gilbert 2014 {published data only}
-
- Armstrong TS, Won M, Wefel JS, Gilbert MR, Pugh SL, Brachman D, et al. Comparative impact of tumor and treatment on patient reported outcomes (PROs) in patients with glioblastoma (GBM) enrolled In RTOG 0825. Journal of Clinical Oncology 2013; Vol. 31 Suppl 15.
-
- Wefel J, Pugh S, Armstrong T, Gilbert M, Won M, Wendland M, et al. Neurocognitive function outcomes in patients with glioblastoma (GBM) enrolled in RTOG 0825. Journal of Clinical Oncology 2013; Vol. 31 Suppl 15.
Herrlinger 2016 {published data only}
-
- Herrlinger U, Schaefer N, Steinbach JP, Weyerbrock A, Hau P, Goldbrunner R, et al. Bevacizumab plus irinotecan versus temozolomide in newly diagnosed O6‐methylguanine–DNA methyltransferase nonmethylated glioblastoma: The Randomized GLARIUS Trial.. Journal of Clinical Oncology 2016;34(14):1611‐19. - PubMed
Lee 2015 {published data only}
-
- Lee EQ, Kaley TJ, Duda DG, Schiff D, Lassman AB, Wong ET, et al. A multicenter, phase II, randomized, noncomparative clinical trial of radiation and temozolomide with or without vandetanib in newly diagnosed glioblastoma patients. Clinical Cancer Research 2015;21(16):3610‐8. [DOI: 10.1158/1078-0432.CCR-14-3220] - DOI - PMC - PubMed
Nabors 2015 {published data only}
-
- Nabors LB, Fink KL, Mikkelsen T, Grujicic D, Tarnawski R, Nam DH, et al. Two cilengitide regimens in combination with standard treatment for patients with newly diagnosed glioblastoma and unmethylated MGMT gene promoter: results of the open‐label, controlled, randomized phase II CORE study. Neuro‐Oncology 2015;17(5):708‐17. - PMC - PubMed
Stupp 2014 {published data only}
-
- Stupp R, Hegi ME, Gorlia T, Erridge S, Perry J, Hong Y‐K, et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071‐22072 study): a multicentre, randomised, open‐label, phase 3 trial. The Lancet Oncology 2014;15:1100‐8. - PubMed
Taal 2014 {published data only}
-
- Taal W, Oosterkamp HM, Walenkamp A, Dubbink H, Beerepoot LV, Hanse M, et al. Single‐agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. The Lancet Oncology 2014;15(9):943‐53. - PubMed
References to studies excluded from this review
Brandes 2016 {published data only}
Duerinck 2016 {published data only}
-
- Duerinck J, Du Four S, Vandervorst S, D'Haene N, Mercier M, Michotte A, et al. Randomized phase II study of axitinib versus physicians best alternative choice of therapy in patients with recurrent glioblastoma. Journal of Neurooncology 2016;128:147‐55. [DOI: 10.1007/s11060-016-2092-2] - DOI - PubMed
Friedman 2009 {published data only}
-
- Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. Journal of Clinical Oncology 2009;27(28):4733‐40. - PubMed
Kreisl 2009 {published data only}
Lombardi 2018 {published data only}
-
- Lombardi G, Salvo GL, Ruda R, Franceschi E, Eoli M, Faedi M, et al. Updated results of REGOMA: A randomized, multicenter, controlled open‐label phase II clinical trial evaluating regorafenib in relapsed glioblastoma (GBM) patients (PTS). Journal of Clinical Oncology. May 2018; Vol. 36, issue 15:2047.
Van Den Bent 2017 {published data only}
-
- Bent M, Klein M, Smits M, Reijneveld JC, Idbaih A, Clement P, et al. Final results of the EORTC Brain Tumor Group randomized phase II TAVAREC trial on temozolomide with or without bevacizumab in 1st recurrence grade II/III glioma without 1p/19q co‐deletion. Journal of Clinical Oncology 2017; Vol. 35 Suppl 15. [DOI: 10.1200/JCO.2017.35.15_suppl.2009] - DOI
References to studies awaiting assessment
Wirsching 2018 {published data only}
-
- Wirsching HG, Tabatabai G, Roelcke U, Hottinger AF, Jörger F, Schmid A, et al. Bevacizumab plus hypofractionated radiotherapy versus radiotherapy alone in elderly patients with glioblastoma: the randomized, open‐label, phase II ARTE trial. Annals of Oncology 10 Apr 2018;29(6):1423‐30. - PubMed
Additional references
Altman 2001
Brandsma 2009
-
- Brandsma D, Bent MJ. Pseudoprogression and pseudoresponse in the treatment of gliomas. Current Opinion in Neurology 2009;22(6):633‐8. - PubMed
CBTRUS 2015
Chinot 2013
Cox 1972
-
- Cox DR. Regression models and life tables. Journal of the Royal Statistical Society 1972;34(2):187‐220.
CTCAE 2017
-
- NIH‐NIC. Common terminology criteria for adverse events (CTCAE v5.0). National Cancer Institute 2017.
Curran 1993
-
- Curran WJ Jr, Scott CB, Horton J, Nelson JS, Weinstein AS, Fischbach AJ, et al. Recursive partitioning analysis of prognostic factors in three radiation therapy oncology group malignant glioma trials. Journal of the National Cancer Institute 1993;85(9):704‐10. - PubMed
DerSimonian 1968
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. - PubMed
Fayers 2001
-
- Fayers PM, Aaronson NK, Bjordal K, Grønvold M, Curran D, Bottomley A, EORTC Quality of Life Group. EORTC QLQ‐C30 Scoring Manual. 3rd Edition. Brussels: European Organisation for Research and Treatment of Cancer, 2001. [ISBN: 2‐9300‐6416‐1]
Fidler 1994
-
- Fidler IJ, Ellis LM. The implications of angiogenesis for the biology and therapy of cancer metastasis . Cell 1994;79(2):185‐8. - PubMed
Field 2015
Field 2017
-
- Field KM, King MT, Simes J, Espinoza D, Barnes EH, Sawkins K, et al. Health‐related quality of life outcomes from CABARET: a randomized phase 2 trial of carboplatin and bevacizumab in recurrent glioblastoma. Journal of Neuro‐oncology 2017;133(3):623‐31. - PubMed
Folkman 1971
-
- Folkman J, Bach M, Rowe JW, Davidoff F, Lambert P, Hirsch C, et al. Tumor angiogenesis ‐ therapeutic implications. New England Journal of Medicine 1971;285(21):1182‐6. - PubMed
Folkman 1990
-
- Folkman J. What is the evidence that tumors are angiogenesis dependent?. Journal of the National Cancer Institute 1990;82(1):4‐6. - PubMed
Gasparini 2005
-
- Gasparini G, Longo R, Fanielli M, Teicher BA. Combination of antiangiogenic therapy with other anticancer therapies: results, challenges and open questions. Journal of Clinical Oncology 2005;23(6):1295‐311. - PubMed
Hicklin 2005
-
- Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. Journal of Clinical Oncology 2005;23(5):1011‐27. - PubMed
Higgins 2003
Higgins 2011a
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
-
- Higgins JP, Deeks JJ, editor(s). Chapter 7: Selecting studies and collecting data. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Kerbel 2008
Khasraw 2010
-
- Khasraw M, Lassman A. Advances in the treatment of malignant gliomas. Current Oncology Reports 2010;12(1):26‐33. - PubMed
Langendam 2013
Lexchin 2003
Lombardi 2017
-
- Lombardi G, Pambuku A, Bellu L, Farina M, Della Puppa A, Denaro l, et al. Effectiveness of antiangiogenic drugs in glioblastoma patients: A systematic review and meta‐analysis of randomized clinical trials. Critical Reviews in Oncology/Hematology 2017;111:94‐102. - PubMed
Louis 2016
Macdonald 1990
-
- Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG. Response criteria for Phase II studies of supratentorial malignant gliomas. Journal of Clinical Oncology 1990;8(7):1277‐80. - PubMed
Machin 1997
-
- Machin D, Stenning SP, Parmar MKP. Thirty years of Medical Research Council randomized trials in solid tumors. Journal of Clinical Oncology 1997;9:100‐14. - PubMed
Mauer 2008
-
- Mauer ME, Bottomley A, Taphoorn MJ. Evaluating health‐related quality of life and symptom burden in brain tumour patients: instruments for use in clinical trials and clinical practice. Current Opinion in Neurology 2008;21:741‐53. - PubMed
Meader 2014
Mirimanoff 2006
-
- Mirimanoff R‐O, Gorlia T, Mason W, Bent MJ, Kortmann R‐D, Fisher B, et al. Radiotherapy and temozolomide for newly diagnosed glioblastoma: recursive partitioning analysis of the EORTC 26981/22981‐NCIC CE3 phase III randomized trial. Journal of Clinical Oncology 2006;24(16):2563‐9. - PubMed
Parmar 1998
-
- Parmar MKB, Torri V, Steward L. Extracting summary statistics to perform meta‐analysis of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815‐34. - PubMed
Radbruch 2011
Review Manager 2014 [Computer program]
-
- Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Scaringi 2013
Sivakumar 2005
-
- Sivakumar B, Harry LE, Paleolog EM. Modulating angiogenesis. JAMA 2005;292(8):972‐7. - PubMed
Stupp 2005
-
- Stupp R, Mason WP, Bent MJ, Weller M, Fisher B, Taphoorn MJB, et al: European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups and the National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New England Journal of Medicine 2005;352:987‐96. - PubMed
Stupp 2007
-
- Stupp R, Hegi ME, Gilbert MR, Chakravarti A. Chemoradiotherapy in malignant glioma: standard of care and future directions. Journal of Clinical Oncology 2007;25(26):4127‐36. - PubMed
Stupp 2009
-
- Stupp R, Hegi ME, Mason WP, Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5‐year analysis of the EORTC‐NCIC trial. Lancet Oncology 2009;10(5):459‐66. - PubMed
Taphoorn 2010
-
- Taphoorn MJB, Claassens L, Aaronson NK, Coens C, Mauer M, Osoba D, et al. An international validation study of the EORTC brain cancer module (EORTC QLQ‐BN20) for assessing health‐related quality of life and symptoms in brain cancer patients. European Journal of Cancer 2010;46(6):1033‐40. - PubMed
Therasse 2000
-
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. Journal of the National Cancer Institute 2000;92(3):205‐16. - PubMed
Vredenburgh 2010
Wen 2010
-
- Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, et al. Updated Response Assessment Criteria for High‐Grade Gliomas: Response Assessment in Neuro‐Oncology Working Group. Journal of Clinical Oncology 2010;28(11):1963‐72. - PubMed
Yan 2009
Zebrowski 1999
-
- Zebrowski BK, Yano S, Liu WB, Shaheen RM, Hicklin DJ, Putnam JB, et al. Vascular endothelial growth factor levels and induction of permeability in malignant pleural effusions. Clinical Cancer Research 1999;5(11):3364‐68. - PubMed
References to other published versions of this review
Khasraw 2010b
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

