Immunogenicity and safety of intramuscular versus subcutaneous administration of a combined measles, mumps, rubella, and varicella vaccine to children 12 to 18 months of age
- PMID: 30481110
- PMCID: PMC6605874
- DOI: 10.1080/21645515.2018.1549452
Immunogenicity and safety of intramuscular versus subcutaneous administration of a combined measles, mumps, rubella, and varicella vaccine to children 12 to 18 months of age
Abstract
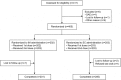
This randomized trial conducted in France compared intramuscular (IM) and subcutaneous (SC) administration of two doses of a measles, mumps, rubella, and varicella (MMRV) combination vaccine (ProQuad®) administered one month apart to 405 children 12-18 months of age (NCT00402831). The 2-dose regimen of MMRV administered IM was shown to be as immunogenic as the 2-dose regimen administered SC for all antigens 6 weeks post-vaccination for the subjects who were initially seronegative for measles, mumps, rubella, or varicella (lower bounds of the two-sided 95% CIs for the difference in response rates for all antigens greater than -10% [range -2.1 for varicella to -3.0 for mumps]). The antibody response rates for all vaccine antigens 6 weeks after the second dose of MMRV were > 99% in both the IM and SC groups. Fewer subjects in the IM group experienced injection-site AEs compared with the SC group (17.8% and 28.6% post-dose 1, and 20.4% and 29.5% post-dose 2, respectively). From Day 0 to Day 4 post-dose 2, fewer subjects reported erythema and swelling in the IM group than in the SC group (15.4% and 27.0%, and 6.0% and 12.5%, respectively). In both groups, most injection-site AEs started during the first four days after vaccination; their intensity was mainly mild or ≤2.5 cm. The rates of fever were comparable between the two groups after each dose of MMRV. In conclusion, two doses of the MMRV vaccine were highly immunogenic and well tolerated when administered either SC or IM. ClinicalTrials.gov Identifier: NCT00402831.
Keywords: IM; Measles; ProQuad; SC; mumps; route of administration; rubella; varicella vaccine.
Similar articles
-
Use of combination measles, mumps, rubella, and varicella vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP).MMWR Recomm Rep. 2010 May 7;59(RR-3):1-12. MMWR Recomm Rep. 2010. PMID: 20448530
-
Immunogenicity and safety of concomitant administration of a measles, mumps and rubella vaccine (M-M-RvaxPro) and a varicella vaccine (VARIVAX) by intramuscular or subcutaneous routes at separate injection sites: a randomised clinical trial.BMC Med. 2009 Apr 14;7:16. doi: 10.1186/1741-7015-7-16. BMC Med. 2009. PMID: 19366435 Free PMC article. Clinical Trial.
-
The immunogenicity and safety of a tetravalent measles-mumps-rubella-varicella vaccine when co-administered with conjugated meningococcal C vaccine to healthy children: A phase IIIb, randomized, multi-center study in Italy.Vaccine. 2016 Aug 5;34(36):4278-84. doi: 10.1016/j.vaccine.2016.07.009. Epub 2016 Jul 14. Vaccine. 2016. PMID: 27423382 Clinical Trial.
-
A combination vaccine against measles, mumps, rubella and varicella.Drugs Today (Barc). 2008 Apr;44(4):279-92. doi: 10.1358/dot.2008.44.4.1210755. Drugs Today (Barc). 2008. PMID: 18536786 Review.
-
A new combination vaccine for measles, mumps, rubella and varicella.Drugs Today (Barc). 2006 May;42(5):321-9. doi: 10.1358/dot.2006.42.5.973586. Drugs Today (Barc). 2006. PMID: 16801995 Review.
Cited by
-
Subcutaneous vaccine administration - an outmoded practice.Hum Vaccin Immunother. 2021 May 4;17(5):1329-1341. doi: 10.1080/21645515.2020.1814094. Epub 2020 Sep 29. Hum Vaccin Immunother. 2021. PMID: 32991241 Free PMC article. Review.
References
-
- World Health Organisation Global measles and rubella strategic plan 2012 – 2020 2012. [accessed 2016 December 6]. https://www.unicef.org/immunization/files/Measles_Rubella_StrategicPlan_....
-
- World Health Organisation Varicella and herpes zoster vaccines: WHO position paper, June 2014. Wkly Epidemiol Rec. 2014;89:265–287. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical