Discovery platform for inhibitors of IgH gene enhancer activity
- PMID: 30481117
- PMCID: PMC6422521
- DOI: 10.1080/15384047.2018.1538615
Discovery platform for inhibitors of IgH gene enhancer activity
Abstract
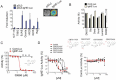
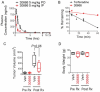
Immunoglobulin heavy chain (IgH) translocations are common and early oncogenic events in B cell and plasma cell malignancies including B cell non-Hodgkin's lymphoma (NHL) and multiple myeloma (MM). IgH translocations bring oncogenes into close proximity with potent enhancer elements within the IgH locus, leading to oncogene up-regulation. As IgH enhancer activity is tightly controlled by B cell lineage-specific signaling and transcriptional networks, we hypothesized that IgH enhancers are potentially druggable targets/elements. To test this, we developed a molecular imaging-based high-throughput screening platform for discovering inhibitors of IgH enhancer-driven transcriptional activity. As proof of concept, we identified a low micromolar potency molecule (compound 30666) that inhibited immunoglobulin production by MM cells and blocked expression of an array of IgH translocation-induced oncogenes (CCND1, FGFR3/MMSET, and MYC) in MM and NHL cell lines. Prolonged exposure to 30666 significantly reduced the viability of IgH translocation-positive NHL and MM cells, but was less effective against cells lacking IgH translocations. Compound 30666 exhibited suitable pharmacological properties, including metabolic stability in liver microsomes and oral bioavailability in mice, and demonstrated preclinical anti-MM activity in a plasmacytoma mouse model. Our work suggests that IgH enhancers are attractive and potentially druggable targets for IgH translocation driven malignancies.
Keywords: IgH Gene Translocation; Multiple myeloma; cancer biology; cytogenetic; gene enhancer; immunoglobulin; non-hodgkins lymphoma; oncogene.
Figures


Similar articles
-
Core regions in immunoglobulin heavy chain enhancers essential for survival of non-Hodgkin lymphoma cells are identified by a CRISPR interference screen.Haematologica. 2024 Dec 1;109(12):4007-4020. doi: 10.3324/haematol.2023.284672. Haematologica. 2024. PMID: 38934080 Free PMC article.
-
7-[[(4-methyl-2-pyridinyl)amino](2-pyridinyl)methyl]-8-quinolinol (compound 30666) inhibits enhancer activity and reduces B-cell lymphoma growth - A question of specificity.Eur J Pharmacol. 2021 Nov 5;910:174505. doi: 10.1016/j.ejphar.2021.174505. Epub 2021 Sep 14. Eur J Pharmacol. 2021. PMID: 34534532
-
Mouse Models of c-myc Deregulation Driven by IgH Locus Enhancers as Models of B-Cell Lymphomagenesis.Front Immunol. 2020 Jul 23;11:1564. doi: 10.3389/fimmu.2020.01564. eCollection 2020. Front Immunol. 2020. PMID: 32793219 Free PMC article. Review.
-
Concomitant downregulation of IgH 3' enhancer activity and c-myc expression in a plasmacytoma x fibroblast environment: implications for dysregulation of translocated c-myc.Mol Immunol. 1997 Feb;34(2):97-107. doi: 10.1016/s0161-5890(97)00017-5. Mol Immunol. 1997. PMID: 9188842
-
Distinguishing primary and secondary translocations in multiple myeloma.DNA Repair (Amst). 2006 Sep 8;5(9-10):1225-33. doi: 10.1016/j.dnarep.2006.05.012. Epub 2006 Jul 10. DNA Repair (Amst). 2006. PMID: 16829212 Review.
Cited by
-
Genome Instability in Multiple Myeloma: Facts and Factors.Cancers (Basel). 2021 Nov 26;13(23):5949. doi: 10.3390/cancers13235949. Cancers (Basel). 2021. PMID: 34885058 Free PMC article. Review.
-
Core regions in immunoglobulin heavy chain enhancers essential for survival of non-Hodgkin lymphoma cells are identified by a CRISPR interference screen.Haematologica. 2024 Dec 1;109(12):4007-4020. doi: 10.3324/haematol.2023.284672. Haematologica. 2024. PMID: 38934080 Free PMC article.
-
Exploring the genetic and molecular basis of differences in multiple myeloma of individuals of African and European descent.Cell Death Differ. 2024 Jan;31(1):1-8. doi: 10.1038/s41418-023-01236-8. Epub 2023 Nov 24. Cell Death Differ. 2024. PMID: 38001255 Free PMC article. Review.
-
Enhancing B-Cell Malignancies-On Repurposing Enhancer Activity towards Cancer.Cancers (Basel). 2021 Jun 29;13(13):3270. doi: 10.3390/cancers13133270. Cancers (Basel). 2021. PMID: 34210001 Free PMC article. Review.
References
-
- Bernicot I, Douet-Guilbert N, Le Bris MJ, Morice P, Abgrall JF, Berthou C, Morel F, De Braekeleer M. Characterization of IGH rearrangements in non-Hodgkin’s B-cell lymphomas by fluorescence in situ hybridization. Anticancer Res. 2005;25:3179–3182. - PubMed
-
- Hayman SR, Bailey RJ, Jalal SM, Ahmann GJ, Dispenzieri A, Gertz MA, Greipp PR, Kyle RA, Lacy MQ, Rajkumar SV, et al. Translocations involving the immunoglobulin heavy-chain locus are possible early genetic events in patients with primary systemic amyloidosis. Blood. 2001;98:2266–2268. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
