Splicing factor SRSF1 promotes gliomagenesis via oncogenic splice-switching of MYO1B
- PMID: 30481162
- PMCID: PMC6355305
- DOI: 10.1172/JCI120279
Splicing factor SRSF1 promotes gliomagenesis via oncogenic splice-switching of MYO1B
Abstract
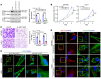
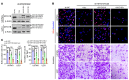
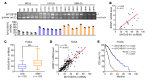
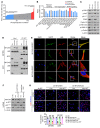
Abnormal alternative splicing (AS) caused by alterations to splicing factors contributes to tumor progression. Serine/arginine splicing factor 1 (SRSF1) has emerged as a key oncodriver in numerous solid tumors, leaving its roles and mechanisms largely obscure in glioma. Here, we demonstrate that SRSF1 is increased in glioma tissues and cell lines. Moreover, its expression was correlated positively with tumor grade and Ki-67 index, but inversely with patient survival. Using RNA-Seq, we comprehensively screened and identified multiple SRSF1-affected AS events. Motif analysis revealed a position-dependent modulation of AS by SRSF1 in glioma. Functionally, we verified that SRSF1 promoted cell proliferation, survival, and invasion by specifically switching the AS of the myosin IB (MYO1B) gene and facilitating the expression of the oncogenic and membrane-localized isoform, MYO1B-fl. Strikingly, MYO1B splicing was dysregulated in parallel with SRSF1 expression in gliomas and predicted the poor prognosis of the patients. Further investigation revealed that SRSF1-guided AS of the MYO1B gene increased the tumorigenic potential of glioma cells through the PDK1/AKT and PAK/LIMK pathways. Taken together, we identify SRSF1 as an important oncodriver that integrates AS control of MYO1B into promotion of gliomagenesis and represents a potential prognostic biomarker and target for glioma therapy.
Keywords: Brain cancer; Cell Biology; Oncology; RNA processing.
Conflict of interest statement
Figures


Similar articles
-
Eucalyptal A inhibits glioma by rectifying oncogenic splicing of MYO1B mRNA via suppressing SRSF1 expression.Eur J Pharmacol. 2021 Jan 5;890:173669. doi: 10.1016/j.ejphar.2020.173669. Epub 2020 Oct 22. Eur J Pharmacol. 2021. PMID: 33098832
-
The RNA-binding protein SRSF1 is a key cell cycle regulator via stabilizing NEAT1 in glioma.Int J Biochem Cell Biol. 2019 Aug;113:75-86. doi: 10.1016/j.biocel.2019.06.003. Epub 2019 Jun 11. Int J Biochem Cell Biol. 2019. PMID: 31200124
-
SRSF3-Regulated RNA Alternative Splicing Promotes Glioblastoma Tumorigenicity by Affecting Multiple Cellular Processes.Cancer Res. 2019 Oct 15;79(20):5288-5301. doi: 10.1158/0008-5472.CAN-19-1504. Epub 2019 Aug 28. Cancer Res. 2019. PMID: 31462429 Free PMC article.
-
Posttranscriptional Regulation of Splicing Factor SRSF1 and Its Role in Cancer Cell Biology.Biomed Res Int. 2015;2015:287048. doi: 10.1155/2015/287048. Epub 2015 Jul 26. Biomed Res Int. 2015. PMID: 26273603 Free PMC article. Review.
-
Recent insights into PDGF-induced gliomagenesis.Brain Pathol. 2010 May;20(3):527-38. doi: 10.1111/j.1750-3639.2009.00335.x. Epub 2009 Sep 25. Brain Pathol. 2010. PMID: 19832839 Free PMC article. Review.
Cited by
-
NONO Inhibits Lymphatic Metastasis of Bladder Cancer via Alternative Splicing of SETMAR.Mol Ther. 2021 Jan 6;29(1):291-307. doi: 10.1016/j.ymthe.2020.08.018. Epub 2020 Sep 5. Mol Ther. 2021. PMID: 32950106 Free PMC article.
-
New Clues to Prognostic Biomarkers of Four Hematological Malignancies.J Cancer. 2022 May 9;13(8):2490-2503. doi: 10.7150/jca.69274. eCollection 2022. J Cancer. 2022. PMID: 35711821 Free PMC article.
-
miR-193a-5p promotes pancreatic cancer cell metastasis through SRSF6-mediated alternative splicing of OGDHL and ECM1.Am J Cancer Res. 2020 Jan 1;10(1):38-59. eCollection 2020. Am J Cancer Res. 2020. PMID: 32064152 Free PMC article.
-
Extracellular matrix stiffness controls VEGF165 secretion and neuroblastoma angiogenesis via the YAP/RUNX2/SRSF1 axis.Angiogenesis. 2022 Feb;25(1):71-86. doi: 10.1007/s10456-021-09804-7. Epub 2021 Jun 25. Angiogenesis. 2022. PMID: 34170441
-
Cyperotundone promotes chemosensitivity of breast cancer via SRSF1.Front Pharmacol. 2025 Mar 19;16:1510161. doi: 10.3389/fphar.2025.1510161. eCollection 2025. Front Pharmacol. 2025. PMID: 40176901 Free PMC article.
References
-
- Srebrow A, Kornblihtt AR. The connection between splicing and cancer. J Cell Sci. 2006;119(pt 13):2635–2641. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

