Deep-learning-assisted diagnosis for knee magnetic resonance imaging: Development and retrospective validation of MRNet
- PMID: 30481176
- PMCID: PMC6258509
- DOI: 10.1371/journal.pmed.1002699
Deep-learning-assisted diagnosis for knee magnetic resonance imaging: Development and retrospective validation of MRNet
Abstract
Background: Magnetic resonance imaging (MRI) of the knee is the preferred method for diagnosing knee injuries. However, interpretation of knee MRI is time-intensive and subject to diagnostic error and variability. An automated system for interpreting knee MRI could prioritize high-risk patients and assist clinicians in making diagnoses. Deep learning methods, in being able to automatically learn layers of features, are well suited for modeling the complex relationships between medical images and their interpretations. In this study we developed a deep learning model for detecting general abnormalities and specific diagnoses (anterior cruciate ligament [ACL] tears and meniscal tears) on knee MRI exams. We then measured the effect of providing the model's predictions to clinical experts during interpretation.
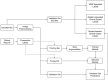
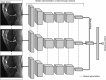
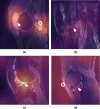
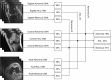
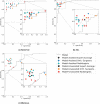
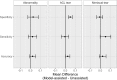
Methods and findings: Our dataset consisted of 1,370 knee MRI exams performed at Stanford University Medical Center between January 1, 2001, and December 31, 2012 (mean age 38.0 years; 569 [41.5%] female patients). The majority vote of 3 musculoskeletal radiologists established reference standard labels on an internal validation set of 120 exams. We developed MRNet, a convolutional neural network for classifying MRI series and combined predictions from 3 series per exam using logistic regression. In detecting abnormalities, ACL tears, and meniscal tears, this model achieved area under the receiver operating characteristic curve (AUC) values of 0.937 (95% CI 0.895, 0.980), 0.965 (95% CI 0.938, 0.993), and 0.847 (95% CI 0.780, 0.914), respectively, on the internal validation set. We also obtained a public dataset of 917 exams with sagittal T1-weighted series and labels for ACL injury from Clinical Hospital Centre Rijeka, Croatia. On the external validation set of 183 exams, the MRNet trained on Stanford sagittal T2-weighted series achieved an AUC of 0.824 (95% CI 0.757, 0.892) in the detection of ACL injuries with no additional training, while an MRNet trained on the rest of the external data achieved an AUC of 0.911 (95% CI 0.864, 0.958). We additionally measured the specificity, sensitivity, and accuracy of 9 clinical experts (7 board-certified general radiologists and 2 orthopedic surgeons) on the internal validation set both with and without model assistance. Using a 2-sided Pearson's chi-squared test with adjustment for multiple comparisons, we found no significant differences between the performance of the model and that of unassisted general radiologists in detecting abnormalities. General radiologists achieved significantly higher sensitivity in detecting ACL tears (p-value = 0.002; q-value = 0.019) and significantly higher specificity in detecting meniscal tears (p-value = 0.003; q-value = 0.019). Using a 1-tailed t test on the change in performance metrics, we found that providing model predictions significantly increased clinical experts' specificity in identifying ACL tears (p-value < 0.001; q-value = 0.006). The primary limitations of our study include lack of surgical ground truth and the small size of the panel of clinical experts.
Conclusions: Our deep learning model can rapidly generate accurate clinical pathology classifications of knee MRI exams from both internal and external datasets. Moreover, our results support the assertion that deep learning models can improve the performance of clinical experts during medical imaging interpretation. Further research is needed to validate the model prospectively and to determine its utility in the clinical setting.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: CL is a shareholder of whiterabbit.ai and nines.ai. Since submitting this manuscript, RLB has joined and received stock options from Roam Analytics, whose mission is to use AI methodology to improve human health.
Figures
References
-
- Nacey NC, Geeslin MG, Miller GW, Pierce JL. Magnetic resonance imaging of the knee: an overview and update of conventional and state of the art imaging. J Magn Reson Imaging. 2017;45:1257–75. 10.1002/jmri.25620 - DOI - PubMed
-
- Naraghi AM, White LM. Imaging of athletic injuries of knee ligaments and menisci: sports imaging series. Radiology. 2016;281:23–40. 10.1148/radiol.2016152320 - DOI - PubMed
-
- Helms CA. Magnetic resonance imaging of the knee In: Brant WE, Helms CA, editors. Fundamentals of diagnostic radiology. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 1193–204.
-
- Oei EH, Nikken JJ, Verstijnen AC, Ginai AZ, Myriam Hunink MG. MR Imaging of the menisci and cruciate ligaments: a systematic review. Radiology. 2003;226:837–48. 10.1148/radiol.2263011892 - DOI - PubMed
-
- Rangger C, Klestil T, Kathrein A, Inderster A, Hamid L. Influence of magnetic resonance imaging on indications for arthroscopy of the knee. Clin Orthop Relat Res. 1996;330:133–42. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

