The Effect of Preexisting Immunity on Virus Detection and Immune Responses in a Phase II, Randomized Trial of a Russian-Backbone, Live, Attenuated Influenza Vaccine in Bangladeshi Children
- PMID: 30481269
- PMCID: PMC6695513
- DOI: 10.1093/cid/ciy1004
The Effect of Preexisting Immunity on Virus Detection and Immune Responses in a Phase II, Randomized Trial of a Russian-Backbone, Live, Attenuated Influenza Vaccine in Bangladeshi Children
Abstract
Background: In a 2012 Phase II clinical trial, 300 Bangladeshi children aged 24 to 59 months with no prior influenza vaccine exposure were randomized to receive a single intranasally-administered dose of either trivalent, Russian-backbone, live, attenuated influenza vaccine (LAIV) or placebo. Protocol-defined analyses, presented in the companion manuscript, demonstrate decreased viral detection and immunogenicity for A/H1N1pdm09, relative to the A/H3N2 and B strains. This post hoc analysis of the trial data aims to investigate the LAIV strain differences by testing the hypothesis that preexisting humoral and mucosal immunity may influence viral recovery and immune responses after LAIV receipt.
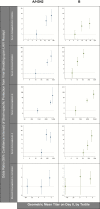
Methods: We used logistic regressions to evaluate the relations between markers of preexisting immunity (ie, hemagglutination inhibition [HAI], microneutralization, and immunoglobulin G and immunoglobulin A (both serum and mucosal antibodies) and LAIV viral recovery in the week post-vaccination. We then tested for potential effect modification by baseline HAI titers (ie, <10 versus ≥10) and week 1 viral recovery on the LAIV-induced serum and mucosal immune responses, measured between days 0 and 21 post-vaccination.
Results: Higher levels of preexisting immunity to influenza A/H3N2 and B were strongly associated with strain-specific prevention of viral shedding upon LAIV receipt. While evidence of LAIV immunogenicity was observed for all 3 strains, the magnitudes of immune responses were most pronounced in children with no evidence of preexisting HAI and in those with detectable virus.
Conclusions: The results provide evidence for a bidirectional association between viral replication and immunity, and underscore the importance of accounting for preexisting immunity when evaluating virologic and immunologic responses to LAIVs.
Clinical trials registration: NCT01625689.
Keywords: humoral immunity; immunogenicity; influenza vaccine; mucosal immunity.
© The Author(s) 2018. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures




Comment in
-
The Potential of Live, Attenuated Influenza Vaccine for the Prevention of Influenza in Children.Clin Infect Dis. 2019 Aug 16;69(5):795-796. doi: 10.1093/cid/ciy1007. Clin Infect Dis. 2019. PMID: 30517601 No abstract available.
References
-
- Neuzil KM, Bresee JS, de la Hoz F, et al. ; WHO Preferred Product Characteristics for Next-Generation Influenza Vaccines Advisory Group. Data and product needs for influenza immunization programs in low- and middle-income countries: Rationale and main conclusions of the WHO preferred product characteristics for next-generation influenza vaccines. Vaccine 2017; 35:5734–7. - PubMed
-
- Paules CI, Sullivan SG, Subbarao K, Fauci AS. Chasing seasonal influenza - The need for a universal influenza vaccine. N Engl J Med 2018; 378:7–9. - PubMed
-
- Boyce TG, Gruber WC, Coleman-Dockery SD, et al. Mucosal immune response to trivalent live attenuated intranasal influenza vaccine in children. Vaccine 1999; 18:82–8. - PubMed

