Sarcolemmal Complement Membrane Attack Complex Deposits During Acute Rejection of Myofibers in Nonhuman Primates
- PMID: 30481300
- PMCID: PMC6289216
- DOI: 10.1093/jnen/nly106
Sarcolemmal Complement Membrane Attack Complex Deposits During Acute Rejection of Myofibers in Nonhuman Primates
Abstract
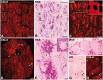
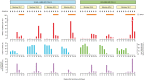
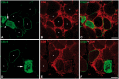
We have previously studied in nonhuman primates several aspects of the acute rejection of myofibers, including the histological characteristics, the mechanisms of myofiber elimination by the T cells, and the development of anti-donor antibodies. Here, we report the participation of the complement membrane attack complex (MAC) in this context. We used muscle sections of macaques from experiments of allogeneic muscle precursor cell transplantation with confirmed rejection of the graft-derived myofibers. Sections were stained with hematoxylin and eosin, alizarin red and for immunodetection of MAC, CD8, CD4, C3, C4d, and immunoglobulins. The prominent finding was the presence of sarcolemmal MAC (sMAC) deposits in biopsies with ongoing acute rejection or with recent acute rejection. The numbers of sMAC-positive myofibers were variable, being higher when there was an intense lymphocyte infiltration. Few sMAC-positive myofibers were necrotic or had evidence of sarcolemma permeation. The immunodetection of C3, C4d, and immunoglobulins did not provide significant elements. In conclusion, sMAC deposits were related to myofiber rejection. The fact that the vast majority of sMAC-positive myofibers had no signs of necrosis or sarcolemmal permeation suggests that MAC would not be harmful to myofibers by itself.
Figures

Similar articles
-
Necrosis, sarcolemmal damage and apoptotic events in myofibers rejected by CD8+ lymphocytes: Observations in nonhuman primates.Neuromuscul Disord. 2012 Nov;22(11):997-1005. doi: 10.1016/j.nmd.2012.05.005. Epub 2012 Jun 29. Neuromuscul Disord. 2012. PMID: 22749896
-
Unexpected sarcolemmal complement membrane attack complex deposits on nonnecrotic muscle fibers in muscular dystrophies.Neurology. 1998 Jan;50(1):41-6. doi: 10.1212/wnl.50.1.41. Neurology. 1998. PMID: 9443455
-
Acute rejection of myofibers in nonhuman primates: key histopathologic features.J Neuropathol Exp Neurol. 2012 May;71(5):398-412. doi: 10.1097/NEN.0b013e31825243ae. J Neuropathol Exp Neurol. 2012. PMID: 22487858
-
Soluble Membrane Attack Complex: Biochemistry and Immunobiology.Front Immunol. 2020 Nov 10;11:585108. doi: 10.3389/fimmu.2020.585108. eCollection 2020. Front Immunol. 2020. PMID: 33240274 Free PMC article. Review.
-
[Immunopathology of acute rejection].G Ital Nefrol. 2005 Nov-Dec;22 Suppl 33:S71-5. G Ital Nefrol. 2005. PMID: 16419010 Review. Italian.
Cited by
-
Establishment of Skeletal Myogenic Progenitors from Non-Human Primate Induced Pluripotent Stem Cells.Cells. 2023 Apr 13;12(8):1147. doi: 10.3390/cells12081147. Cells. 2023. PMID: 37190056 Free PMC article.
References
-
- Vilquin JT, Catelain C, Vauchez K.. Cell therapy for muscular dystrophies: Advances and challenges. Curr Opin Organ Transplant 2011;16:640–9 - PubMed
-
- Skuk D, Tremblay JP.. Cell therapy in muscular dystrophies: Many promises in mice and dogs, few facts in patients. Expert Opin Biol Ther 2015;15:1307–19 - PubMed
-
- Partridge TA. Invited review: myoblast transfer: a possible therapy for inherited myopathies? Muscle Nerve 1991;14:197–212 - PubMed
-
- Skuk D, Goulet M, Roy B, et al. Dystrophin expression in muscles of Duchenne muscular dystrophy patients after high-density injections of normal myogenic cells. J Neuropathol Exp Neurol 2006;65:371–86 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

