Prolonged thromboprophylaxis with low molecular weight heparin for abdominal or pelvic surgery
- PMID: 30481366
- PMCID: PMC6517131
- DOI: 10.1002/14651858.CD004318.pub3
Prolonged thromboprophylaxis with low molecular weight heparin for abdominal or pelvic surgery
Update in
-
Prolonged thromboprophylaxis with low molecular weight heparin for abdominal or pelvic surgery.Cochrane Database Syst Rev. 2019 Mar 27;3(3):CD004318. doi: 10.1002/14651858.CD004318.pub4. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2019 Aug 26;8:CD004318. doi: 10.1002/14651858.CD004318.pub5. PMID: 30916777 Free PMC article. Updated.
Abstract
Background: This an update of the review first published in 2009.Major abdominal and pelvic surgery carries a high risk of venous thromboembolism (VTE). The efficacy of thromboprophylaxis with low molecular weight heparin (LMWH) administered during the in-hospital period is well-documented, but the optimal duration of prophylaxis after surgery remains controversial. Some studies suggest that patients undergoing major abdominopelvic surgery benefit from prolongation of the prophylaxis up to 28 days after surgery.
Objectives: To evaluate the efficacy and safety of prolonged thromboprophylaxis with LMWH for at least 14 days after abdominal or pelvic surgery compared with thromboprophylaxis administered during the in-hospital period only in preventing late onset VTE.
Search methods: We performed electronic searches on 28 October 2017 in the Cochrane Central Register of Controlled Trials, MEDLINE, Embase, LILACS and registered trials (Clinicaltrials.gov October 28, 2017 and World Health Organization International Clinical Trials Registry Platform (ICTRP) 28 October 2017). Abstract books from major congresses addressing thromboembolism were handsearched from 1976 to 28 October 2017, as were reference lists from relevant studies.
Selection criteria: We assessed randomized controlled clinical trials (RCTs) comparing prolonged thromboprophylaxis (≥ fourteen days) with any LMWH agent with placebo, or other methods, or both to thromboprophylaxis during the admission period only. The population consisted of persons undergoing abdominal or pelvic surgery for both benign and malignant pathology. The outcome measures included VTE (deep venous thrombosis (DVT) or pulmonary embolism (PE)) as assessed by objective means (venography, ultrasonography, pulmonary ventilation/perfusion scintigraphy, spiral computed tomography (CT) scan or autopsy). We excluded studies exclusively reporting on clinical diagnosis of VTE without objective confirmation.
Data collection and analysis: Review authors identified studies and extracted data. Outcomes were VTE (DVT or PE) assessed by objective means. Safety outcomes were defined as bleeding complications within three months after surgery. Sensitivity analyses were also performed with unpublished studies excluded, and with study participants limited to those undergoing solely open and not laparoscopic surgery. We used a fixed-effect model for analysis.
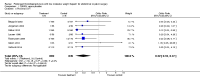
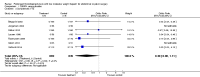
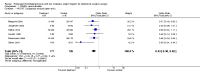
Main results: We identified seven RCTs (1728 participants) evaluating prolonged thromboprophylaxis with LMWH compared with control or placebo. The searches resulted in 1632 studies, of which we excluded 1528. One hundred and four abstracts, eligible for inclusion, were assessed of which seven studies met the inclusion criteria.For the primary outcome, the incidence of overall VTE after major abdominal or pelvic surgery was 13.2% in the control group compared to 5.3% in the patients receiving out-of-hospital LMWH (Mantel Haentzel (M-H) odds ratio (OR) 0.38, 95% confidence interval (CI) 0.26 to 0.54; I2 = 28%; seven studies, n = 1728; moderate-quality evidence).For the secondary outcome of all DVT, seven studies, n = 1728, showed prolonged thromboprophylaxis with LMWH to be associated with a statistically significant reduction in the incidence of all DVT (M-H OR 0.39, 95% CI 0.27 to 0.55; I2 = 28%; moderate-quality evidence).We found a similar reduction when analysis was limited to incidence in proximal DVT (M-H OR 0.22, 95% CI 0.10 to 0.47; I2 = 0%; moderate-quality evidence).The incidence of symptomatic VTE was also reduced from 1.0% in the control group to 0.1% in patients receiving prolonged thromboprophylaxis (M-H OR 0.30, 95% CI 0.08 to 1.11; I2 = 0%; moderate-quality evidence).No difference in the incidence of bleeding between the control and LMWH group was found, 2.8% and 3.4%, respectively (HM-H OR 1.10, 95% CI 0.67 to 1.81; I2 = 0%; seven studies, n = 2239; moderate-quality evidence).Estimates of heterogeneity ranged between 0% and 28% depending on the analysis, suggesting low or unimportant heterogeneity.
Authors' conclusions: Prolonged thromboprophylaxis with LMWH significantly reduces the risk of VTE compared to thromboprophylaxis during hospital admittance only, without increasing bleeding complications after major abdominal or pelvic surgery. This finding also holds true for DVT alone, and for both proximal and symptomatic DVT. The quality of the evidence is moderate and provides moderate support for routine use of prolonged thromboprophylaxis. Given the low heterogeneity between studies and the consistent and moderate evidence of a decrease in risk for VTE, our findings suggest that additional studies may help refine the degree of risk reduction but would be unlikely to significantly influence these findings. This updated review provides additional evidence and supports the previous results reported in the 2009 review.
Conflict of interest statement
MSR is a member the advisory board of Pfizer, Denmark.
One author has been investigators on three of the randomized trials included in this review update (Rasmussen 2006).
Figures

















Update of
-
Prolonged thromboprophylaxis with low molecular weight heparin for abdominal or pelvic surgery.Cochrane Database Syst Rev. 2009 Jan 21;(1):CD004318. doi: 10.1002/14651858.CD004318.pub2. Cochrane Database Syst Rev. 2009. Update in: Cochrane Database Syst Rev. 2018 Nov 27;11:CD004318. doi: 10.1002/14651858.CD004318.pub3. PMID: 19160234 Updated.
Similar articles
-
Prolonged thromboprophylaxis with low molecular weight heparin for abdominal or pelvic surgery.Cochrane Database Syst Rev. 2019 Mar 27;3(3):CD004318. doi: 10.1002/14651858.CD004318.pub4. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2019 Aug 26;8:CD004318. doi: 10.1002/14651858.CD004318.pub5. PMID: 30916777 Free PMC article. Updated.
-
Prolonged thromboprophylaxis with low molecular weight heparin for abdominal or pelvic surgery.Cochrane Database Syst Rev. 2019 Aug 26;8(8):CD004318. doi: 10.1002/14651858.CD004318.pub5. Cochrane Database Syst Rev. 2019. PMID: 31449321 Free PMC article.
-
Prolonged thromboprophylaxis with low molecular weight heparin for abdominal or pelvic surgery.Cochrane Database Syst Rev. 2009 Jan 21;(1):CD004318. doi: 10.1002/14651858.CD004318.pub2. Cochrane Database Syst Rev. 2009. Update in: Cochrane Database Syst Rev. 2018 Nov 27;11:CD004318. doi: 10.1002/14651858.CD004318.pub3. PMID: 19160234 Updated.
-
Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy.Cochrane Database Syst Rev. 2020 Dec 18;12(12):CD008500. doi: 10.1002/14651858.CD008500.pub5. Cochrane Database Syst Rev. 2020. PMID: 33337539 Free PMC article.
-
Interventions for preventing venous thromboembolism in adults undergoing knee arthroscopy.Cochrane Database Syst Rev. 2020 May 6;5(5):CD005259. doi: 10.1002/14651858.CD005259.pub4. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2022 Aug 22;8:CD005259. doi: 10.1002/14651858.CD005259.pub5. PMID: 32374919 Free PMC article. Updated.
Cited by
-
Risk of Pulmonary Embolism More Than 6 Weeks After Surgery Among Cancer-Free Middle-aged Patients.JAMA Surg. 2019 Dec 1;154(12):1126-1132. doi: 10.1001/jamasurg.2019.3742. JAMA Surg. 2019. PMID: 31596449 Free PMC article.
-
Extended thromboprophylaxis for medically ill patients with cancer: a systemic review and meta-analysis.Blood Adv. 2021 Apr 27;5(8):2055-2062. doi: 10.1182/bloodadvances.2020004118. Blood Adv. 2021. PMID: 33861298 Free PMC article.
-
The efficacy and safety of low-molecular-weight heparin calcium combined with Xueshuantong injections in the treatment of elderly acute deep venous thrombosis patients.Am J Transl Res. 2021 Apr 15;13(4):3120-3128. eCollection 2021. Am J Transl Res. 2021. PMID: 34017480 Free PMC article.
-
Rivaroxaban vs placebo for extended antithrombotic prophylaxis after laparoscopic surgery for colorectal cancer.Blood. 2022 Aug 25;140(8):900-908. doi: 10.1182/blood.2022015796. Blood. 2022. PMID: 35580191 Free PMC article. Clinical Trial.
-
American Society of Hematology 2019 guidelines for management of venous thromboembolism: prevention of venous thromboembolism in surgical hospitalized patients.Blood Adv. 2019 Dec 10;3(23):3898-3944. doi: 10.1182/bloodadvances.2019000975. Blood Adv. 2019. PMID: 31794602 Free PMC article.
References
References to studies included in this review
-
- Bergqvist D, Agnelli G, Cohen AT, Eldor A, Nilsson PE, Moigne‐Amrani AL, et al. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. New England Journal of Medicine 2002;346(13):975‐90. - PubMed
-
- Jørgensen LN, Lausen I, Rasmussen MS, Wille‐Jørgensen P, Bergqvist D. Prolonged thromboprophylaxis with low‐molecular weight heparin following major general surgery: an individual patient data meta‐analysis. Blood. 2002; Vol. 100:abstract 1952 (poster).
-
- Kakkar VV, Balibrea JL, Martinez‐Gonzalez J, Prandoni P. Extended prophylaxis with bemiparin for the prevention of venous thromboembolism after abdominal or pelvic surgery for cancer: the CANBESURE randomized study. Journal of Thrombosis and Haemostasis 2010;8(6):1223‐39. - PubMed
-
- Lausen I, Jensen R, Jorgensen LN, Rasmussen MS, Lyng KM, Andersen M, et al. Incidence and prevention of deep venous thrombosis occurring late after general surgery: Randomised controlled study of prolonged thromboprophylaxis. European Journal of Surgery 1998;164:657‐63. - PubMed
-
- Rasmussen MS, Jorgensen LN, Wille‐Jørgensen P, Nielsen JD, Horn A, Mohn AC, et al. Prolonged prophylaxis with dalteparin to prevent late thromboeembolic complications in patients undergoing major abdominal surgery: a multicenter randomised open‐label study. Thrombosis and Haemostasis 2006;4:2384‐90. - PubMed
References to studies excluded from this review
-
- Downs LS. Women's Cancer Center Protocol #45: Prolonged venous thromboembolism prophylaxis with fondaparinux in gynecologic oncology patients: an open label phase ii trial. Clinicaltrials.gov2012.
-
- GlaxoSmithKline. Clinical evaluation of GSK576428 (fondaparinux sodium) in prevention of venous thromboembolism after abdominal surgery. Clinicaltrials.gov July 10, 2009.
-
- Huh W. Extended deep venous thrombosis prophylaxis in gynecologic oncology surgery with intermittent compression devices (ICD) with or without postoperative arixtra (fondaparinux sodium): a randomized controlled trial. Clinicaltrials.gov March 27, 2017.
-
- Krasiński Z, Szpurek D, Staniszewski R, Dzieciuchowicz Ł, Pawlaczyk K, Krasińska B, et al. The value of extended preoperative thromboprophylaxis with dalteparin in patients with ovarian cancer qualified to surgical treatment. International Angiology 2014;33(4):365‐71. - PubMed
References to studies awaiting assessment
-
- Zhang, Z. The mechanical and medical prevention of lower extremity deep venous thrombosis formation post gynecologic pelvic surgery, a multiple center randomized case control study. ChiCTR‐IPR‐15007324 Recruiting.
References to ongoing studies
-
- Campanini M (FADOI Foundation, Italy). Rivaroxaban or placebo for extended antithrombotic prophylaxis after laparoscopic surgery for colorectal cancer: a randomized, double blind, placebo‐controlled study. Clinicaltrials.gov2017.
Additional references
-
- Akay OM, Ustuner Z, Canturk Z, Mutlu FS, Gulbas Z. Laboratory investigation of hypercoagulability in cancer patients using rotation thrombelastography. Medical Oncology 2009;26(3):358‐64. - PubMed
-
- Amin AN, Lin J, Ryan A. Need to improve thromboprophylaxis across the continuum of care for surgical patients. Advances in Therapy 2010;27(2):81‐93. - PubMed
-
- Arnesen H, Dahl OE, Aspelin T, Seljeflot I, Kierulf P, Lyberg T. Sustained prothrombotic profile after hip replacement surgery: the influence of prolonged prophylaxis with dalteparin. Journal of Thrombosis and Haemostasis 2003;1:971‐5. - PubMed
-
- Bahl V, Hu HM, Henke PK, Wakefield TW, Campbell DA Jr, Caprini JA. A validation study of a retrospective venous thromboembolism risk scoring method. Annals of Surgery 2010;251(2):344‐50. - PubMed
References to other published versions of this review
-
- Rasmussen MS, Wille‐Jørgensen P. Prolonged thromboprophylaxis for abdominal surgery. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD004318] - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

